When it comes to starting a stem cell therapy business, you are required to first look at the existing laws in the country or the state you reside because there is hardly any country that does not pay serious attention on their health sector and stem cell therapy business is not left out.
Full Answer
How do you become a stem cell Doctor?
The cost for leasing facility for the human stem cell therapy lab – $180,000. The cost for facility remodeling – $50,000. Other start-up expenses including stationery – $1000. Phone and utility …
Do stem cell clinics work?
This course provides you EVERYTHING you need to know to successfully practice, build and operate a high revenue stem cell and regenerative medicine clinic. You will learn: What …
How to start a stem cell therapy business?
Jul 31, 2017 · Step-by-Step Educational Path to Becoming a Regenerative Stem Cell Doctor. Bachelor's Degree. Holding an undergraduate degree from an accredited college is necessary …
What is stem cell therapy?
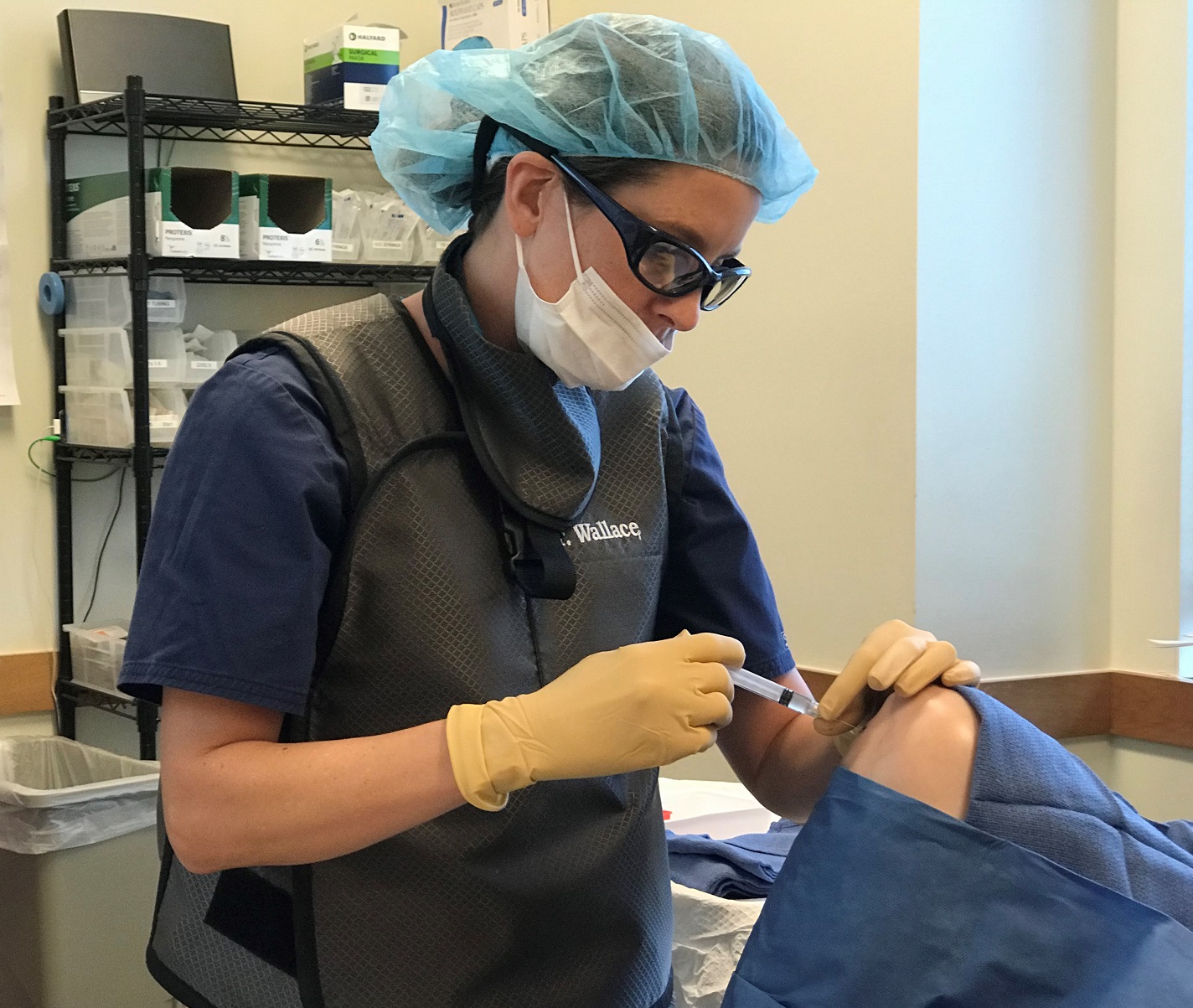
Jun 17, 2019 · Make sure that you are receiving a cell procedure with a high volume of live autologous stem cells. In addition, make sure the injection is being performed by a trained …

How much funding is needed for stem cell research?
Where do doctors get stem cells for stem cell therapy?
What is the cost of stem cell therapy?
How do hospitals get stem cells?
How do you activate stem cells?
Stem cell proliferation is enhanced with exercise. In particular, aerobic exercise has been proven to help stem cells turn into bone instead of fat. Scientists at the University of Illinois have found that stem cells are activated in the muscles during exercise.
Which country has the most advanced stem cell therapy?
| Rank | Country/Territory | Number of clinical trials |
|---|---|---|
| 1 | United States | 136 |
| 2 | Iran | 65 |
| 3 | South Korea | 40 |
| 4 | Australia | 18 |
How successful is stem cell treatment?
How many stem cell injections are needed?
Can you buy stem cells?
Can you grow your own stem cells?
Is stem cell harvesting painful?
What happens after 100 days of stem cell transplant?
How much does it cost to start a stem cell clinic?
You could become a millionaire owning a stem cell practice... You can start a stem cell and regenerative medicine clinic for less than $7,500.
Can you do stem cell injections with ultrasound?
Stem cell injections may be done with or without ultrasound. Ultrasound gives the patient more peace of mind and it also helps to specifically target the desired injection site. Ultrasound also allows the avoidance of hitting veins and/or arteries even though this risk is minimal if you understand proper technique.
Why is it important to have only one stem cell product?
It is also essential that one person works with only one stem cell product at any one time to prevent mix up of samples. If the laboratory is within a hospital, a staff sharing arrangement with the blood bank or other clinical laboratory can be a cost-effective strategy for processing staff backup.
What is a CPL in blood and marrow transplant?
The Graft Processing subcommittee of the Worldwide Network for Blood and Marrow Transplantation wrote this guideline to assist physicians and laboratory technologists with the setting up of a cell processing laboratory (CPL) to support a hematopoietic stem cell transplant program, thereby facilitating the start-up of a transplant program in a new location and improving patient access to transplantation worldwide. This guideline describes the minimal essential features of designing such a laboratory and provides a list of equipment and supply needs and staffing recommendations. It describes the typical scope of services that a CPL is expected to perform, including product testing services, and discusses the basic principles behind the most frequent procedures. Quality management (QM) principles specific to a CPL are also discussed. References to additional guidance documents that are available worldwide to assist with QM and regulatory compliance are also provided.
What is a CPL in a lab?
This report is intended as a guide for laboratory professionals tasked with setting up a cell processing laboratory (CPL) to support a new hematopoietic stem cell transplant program; especially in a region or country with limited resources. It describes the essential features of a laboratory’s design and setup, provides basic processing principles and quality management (QM) guidelines and references additional guidance documents available worldwide. The Graft Processing subcommittee of the Worldwide Network for Blood and Marrow Transplantation 1 wrote this guideline to help physicians and laboratory technologists establish and run a CPL that will support either an autologous or allogeneic hematopoietic stem cell transplant program, thereby improving patient access to transplantation as a potentially curative modality of treatment.
Is a small dedicated space sufficient for a CPL?
When first establishing a transplant program, a relatively small dedicated space or even a clearly defined shared space is typically sufficient for a CPL, provided product safety risks such as cross contamination or product mix-ups are taken into consideration with the design and location.
What is the importance of a sink and water supply in a laboratory?
A sink and water supply for hand washing and cleaning within the laboratory or nearby is important. Dedicated workstation (s) in a clean environment with restricted controlled access and away from potential contaminants are essential.
What is the importance of CPL?
Education and training of staff is critical for the establishment and operation of a CPL. Processing products for cryopreservation is not a highly technical operation; however, attention to detail, strict aseptic technique, a quality focus and consideration of the importance of each product for the patient are key factors for success. Staff with formal education in a laboratory-based discipline, and preferably with some experience in clinical hematology and/or blood banking, are most suited for the work that is required.
Is cryopreservation a technical process?
Processing products for cryopreservation is not a highly technical operation; however, attention to detail, strict aseptic technique, a quality focus and consideration of the importance of each product for the patient are key factors for success.
What is stem cell therapy?
Overview. A new frontier in medicine, regenerative medicine or stem cell therapy involves the study of or the application of stem cells as a form of healing. Stem cells are naturally reproduced in the body therefore they are a renewable source of medicine with enormous potential. Still a relatively new area of scientific study, ...
Is stem cell a renewable source?
Stem cells are naturally reproduced in the body therefore they are a renewable source of medicine with enormous potential. Still a relatively new area of scientific study, it is unknown just how far and wide reaching the healing capabilities of this type of therapy will be, but the potential seems quite limitless.
What is a regenerative stem cell doctor?
A regenerative stem cell doctor is a specialist who uses stem cell therapy to treat patients. As this is still a comparatively new form of medicine, this type of doctor will likely be involved in research or clinical trials, discovering new treatments and their effects as well as preventative approaches based on cellular technology ...
What are the three types of stem cells?
Stem cells generally fall under three categories: Embryonic stem cells. Adult stem cells. Stem cells from other organisms. A stem cell doctor may use this type of medical treatment with patients who suffer from autoimmune deficiencies or genetic blood diseases. It's also typical for stem cell doctors to focus on pharmaceutical product development ...
What is embryonic stem cell?
Embryonic stem cells. Adult stem cells. Stem cells from other organisms. A stem cell doctor may use this type of medical treatment with patients who suffer from autoimmune deficiencies or genetic blood diseases. It's also typical for stem cell doctors to focus on pharmaceutical product development in areas of systemic diseases, ...
What is stem cell doctor?
A stem cell doctor may use this type of medical treatment with patients who suffer from autoimmune deficiencies or genetic blood diseases. It's also typical for stem cell doctors to focus on pharmaceutical product development in areas of systemic diseases, cardiac and vascular diseases, ...
What is the best background for stem cell medicine?
For those whose sights are set on eventually becoming a medical scientist and working in the field of regenerative stem cell medicine, it is ideal to begin with a strong background in biology, chemistry and biophysics. Majoring in life science, biology, microbiology or pre-med is a good starting point as well as taking writing classes as this field ...
How to get a full picture of a patient's experience with the clinic?
To get a full picture of what a patient’s experience with the clinic may be like, read into detailed stem cell patient case studies. Remember that success is subjective and the treatment should start with a thorough evaluation of your imaging and symptoms. An experienced stem cell physician will make sure that the patient is not misinformed by ...
What is stem cell procedure?
The term “stem cell procedure” has become a catchphrase for the use of just about any type of biologic injection, regardless of whether the injection contains live stem cells or not. There is now a constant stream of advertising for stem cell treatments for nearly every medical problem imaginable.
Does bone marrow have a regenerative effect?
The cell counts in bone marrow fall dramatically as a patient ages; and after the age of 40 cell counts may not be adequate to provide an appreciative regenerative effect when used alone. This is not the case with adipose-derived cells, where cell counts remain significant throughout a patient’s life.
How many times more cells are in a good fat harvest than bone marrow?
There are 500-2000 times more cells in a good fat harvest than are found in bone marrow concentrate. The use of adipose derived cells offers a much greater regenerative potential than bone marrow concentrate. The two autologous cell sources can be used in combination to maximize healing potential.
Should you compare the price of stem cell surgery?
It is imperative to not simply compare the price of a stem cell procedure when making the decision on the appropriate regenerative stem cell treatment. Be selective when looking at ortho-biologic options. Make sure that you are receiving a cell procedure with a high volume of live autologous stem cells.
Where do stem cells come from?
Stem cells are sourced from a combination of both fat and bone marrow, harvested from your own body. Detailed case studies about the clinic’s own patients. Patient reviews.
Where are stem cells found?
In mammals, there are two broad types of stem cells: embryonic stem cells, which are isolated from the inner cell mass of blastocysts, and adult stem cells, which are found in various body tissues. In adult organisms, stem cells ...
What are the two types of stem cells?
In mammals, there are two broad types of stem cells: embryonic stem cells, which are isolated from the inner cell mass of blastocysts, and adult stem cell s, which are found in various body tissues. In adult organisms, stem cells and progenitor cells act as a repair system for the body, replenishing adult tissues.
How much money did stem cell technology make in 2016?
Stem cell technology attracts the vast majority of investment; $700 million of the $800 million dedicated to regenerative medicine in 2016 went to stem cell projects. This shows that the stem cell industry, though budding, has a huge potential for profits because it is concerned with life, health and wellness.
Is stem cell regenerative a new technology?
Stem cell transplantation and the general stem cell regenerative industry is a relatively new technology ...
Is stem cell storage capital intensive?
The storage of stem cells, usually in the form of cord blood, is a growing industry, and an entrepreneur that has some knowledge of the industry can come in now and make profits. This business may be capital intensive because you need to build a suitable bank that can help you to properly store this blood product.
What is stem cell therapy?
Cell therapy, according to the National Institutes of Health (NIH), is defined as the process of creating living, functional tissues to repair or replace tissue or organ function loss due to age, disease, damage or congenital defects.
Is stem cell therapy effective for wound healing?
Stem cell therapy has been known to be effective in healing chronic wounds, and quite a number of products have been developed to that effect.
What are the different types of stem cell therapy?
Let’s start by creating two categories of stem cell therapies – approved (by the FDA) and unapproved. Whether a stem cell therapy is approved or unapproved has critical implications for the science, effectiveness, and safety of the procedure.
How long does it take for stem cell therapy to work?
Furthermore, there is no proof that any stem cell therapy offered by stem cell clinics is effective or safe. Unlike FDA-approved procedures, which are subject to years of rigorous trials, unapproved treatments marketed directly to patients are developed and performed with little oversight.
What are the negative effects of stem cell therapy?
There are side effects associated with approved and unapproved stem cell therapies. The possible side effects of blood stem cell transplants are detailed on the Cancer.org website.
How long does stem cell therapy last?
In their advertising, stem cell clinics promise unsubstantiated relief or even cures for everything from knee pain to Parkinson’s disease, often taking advantage of vulnerable individuals who may feel they have nowhere else to turn.

Laboratory Design and Physical Plant Requirements
- When first establishing a transplant program, a relatively small dedicated space or even a clearly defined shared space is typically sufficient for a CPL, provided product safety risks such as cross contamination or product mix-ups are taken into consideration with the design and location. The laboratory should be located as far from potential cont...
Equipment
- The equipment requirements for a CPL are fairly minimal. Table 1 lists the equipment needed in three categories: Required, Desirable, (nice to have), and Shared (equipment that because of expense, maintenance considerations and low volume use could be shared with another laboratory within reasonable proximity). Critical equipment should be maintained and calibrated …
Supplies and Reagents
- A partial list of supplies and reagents that will be needed is provided in Table 2. All product contact reagents need to be sterile and of infusion-grade. All supplies need to be sterile and disposable; used once and discarded. Reagents can be dispensed into single-use containers before use in order to minimize waste. All reagents and supplies need to be inspected before us…
Personnel
- Education and training of staff is critical for the establishment and operation of a CPL. Processing products for cryopreservation is not a highly technical operation; however, attention to detail, strict aseptic technique, a quality focus and consideration of the importance of each product for the patient are key factors for success. Staff with formal education in a laboratory-based discipli…
Processing Principles
- The CPL’s primary role in supporting an autologous transplant program is to preserve cellular therapy product viability during storage and to prevent the introduction of microbial contamination at all stages of processing. The autograft is collected before the patient receives high-dose therapy,5and depending on the disease and treatment plan, cellular therapy products may need t…
Cellular Therapy Product Characterization/Quality Control Testing
- BM contains a wide range of cell numbers and cell types and responses to agents used to mobilize HPCs to the peripheral blood vary, especially for the autologous donor. Therefore, it is imperative that the laboratory provide the patient’s physician with cell count data and CD34+ cell count data so they can assess the potential of the cellular therapy product to engraft before initi…
Product Release
- Standard operating procedures or policies must be in place defining the criteria that must be met for a product to be released from the laboratory for infusion. Staff releasing the product must confirm that all criteria are met; or if they are not met, that the required approvals and notifications have been obtained from the transplant physician or Medical Director. Ideally, final …
Quality Plan Essentials
- Finally, each of us wishes to emphasize the importance of starting the planning for building a processing laboratory by writing a Quality Plan specific to the laboratory. A quality plan summarizes and references the organization’s policies, procedures and practices related to the day-to-day operation of the facility and in the case of emergency. There are many resources avai…
Regulatory Compliance
- Regulatory compliance requirements will vary greatly between countries. In general, a solid quality plan that addresses all local regulations, adherence to the principles of that plan and documentation to support that adherence will likely allow the laboratory to comply with the regulatory frameworks. FACT, AABB, JACIE, Netcord, The Alliance for Harmonization of Cellular …