Common question
What is convalescent plasma for the treatment of COVID-19?Convalescent plasma (kon-vuh-LES-unt PLAZ-muh) therapy uses blood from people who've recovered from an illness to help others recover. The U.S. Food and Drug Administration (FDA) has given emergency authorization for convalescent plasma therapy with high antibody levels to treat COVID-19 .Apr 28, 2021
Explore
Convalescent plasma is a treatment therapy that provides passive immunity for someone who has an illness for the first time. Healthcare providers may use convalescent plasma therapy when there are new viral outbreaks, such as COVID-19.
How effective is convalescent plasma?
Sep 03, 2020 · What is convalescent plasma? Convalescent plasma is human plasma collected from individuals who have recovered from an infection. How does convalescent plasma treatment work? In the case of COVID-19 infection, convalescent plasma should contain anti-SARS-CoV-2 antibodies. Antibodies are special proteins that the body generates to fight infection.
Should I donate convalescent plasma?
Jan 18, 2022 · Antibody therapy in the form of convalescent plasma, also known as serum therapy, goes back 130 years. We have a lot of experience transferring antibodies to treat diseases. Whenever there is a pandemic or an epidemic, physicians often resort to it, because it’s available as soon as a person has recovered.
Who can donate convalescent plasma?
Apr 18, 2020 · Convalescent plasma is the cell-free liquid part of blood (plasma) drawn from people who have recovered (convalesced) from COVID-19. It contains antibodies that help fight the virus off. We’ve used convalescent plasma to treat serious infections for nearly 100 years. So far, we only have small studies of convalescent plasma treatment for COVID-19.
Does CSL or Biolife pay more?
Aug 25, 2020 · Convalescent means recovering, so this term refers to plasma sourced from people who’ve recovered from COVID-19. When someone gets sick with — and generates antibodies for — the novel coronavirus, those antibodies remain in their plasma (the liquid part of blood) for an unknown period of time.
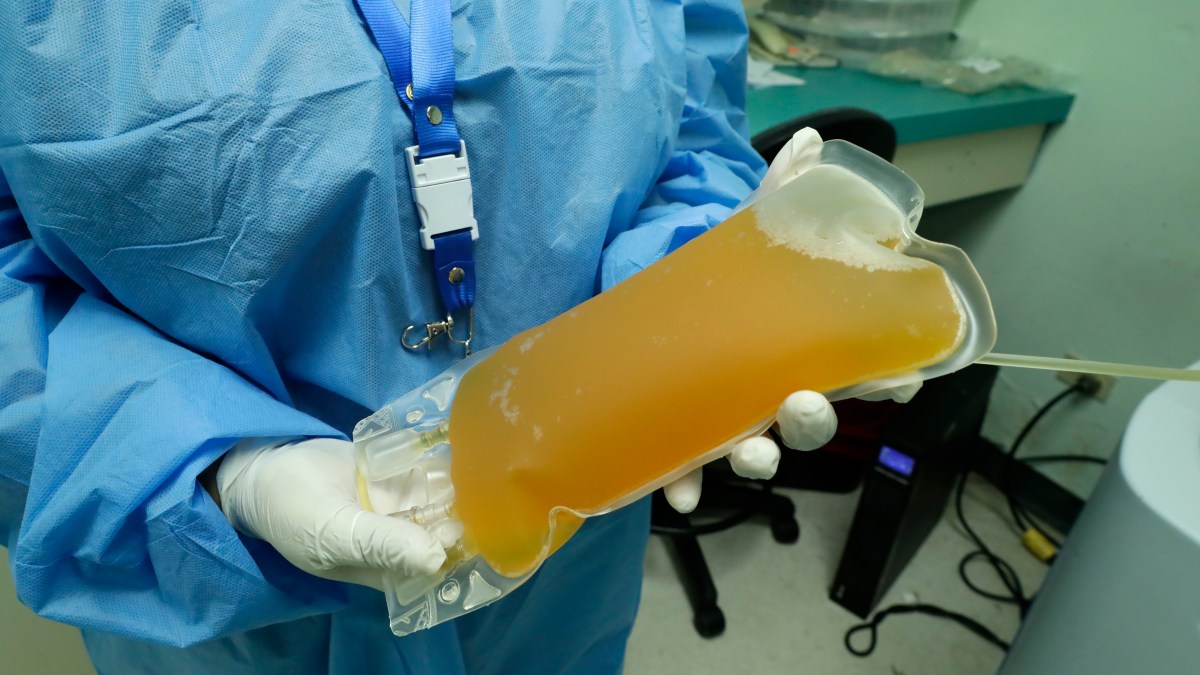
How can convalescent plasma be used to treat COVID-19?
The blood from people who recover from COVID-19 contains substances called antibodies, which are capable of fighting the virus that causes the illness. For some other diseases caused by respiratory viruses, giving people the liquid portion of blood that contains these antibodies, called plasma, obtained from those who have recovered from the virus, may lead to more rapid improvement of the disease. Patients with COVID-19 may improve faster if they receive plasma from those who have recovered from COVID-19, because it may have the ability to fight the virus that causes COVID-19.Dec 28, 2021
Can you get COVID-19 if you already had it and have antibodies?
It is important to remember that some people with antibodies to SARS-CoV-2 may become infected after vaccination (vaccine breakthrough infection) or after recovering from a past infection (reinfected).Nov 10, 2021
Can you get the Covid vaccine if you were treated with convalescent plasma?
If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine. Talk to your doctor if you are unsure what treatments you received or if you have more questions about getting a COVID-19 vaccine.
What is the treatment for COVID-19?
Clinical trials are looking into whether some drugs and treatments used for other conditions might treat severe COVID-19 or related pneumonia, including dexamethasone, a corticosteroid. The FDA has approved the antiviral remdesivir (Veklury) for treatment of patients hospitalized with COVID.Jan 25, 2022
How long do COVID-19 antibodies last?
At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity.Jan 31, 2022
How long does it take to develop immunity after a COVID-19 infection?
Although the immune correlates of protection are not fully understood, evidence indicates that antibody development following infection likely confers some degree of immunity from subsequent infection for at least 6 months.
Should you still get the COVID-19 vaccine if you were treated with monoclonal antibodies?
If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, there is no need to delay getting a COVID-19 vaccine.Feb 17, 2022
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
What medication is not recommended before vaccinations for COVID-19?
It is not recommended you take over-the-counter medicine – such as ibuprofen, aspirin, or acetaminophen – before vaccination for the purpose of trying to prevent vaccine-related side effects. It is not known how these medications might affect how well the vaccine works.
Which medications can help reduce the symptoms of COVID-19?
In terms of specifics: acetaminophen (Tylenol), naproxen (Aleve) or ibuprofen (Advil, Motrin) can help lower your fever, assuming you don't have a health history that should prevent you from using them. It's usually not necessary to lower a fever – an elevated temperature is meant to help your body fight off the virus.Dec 21, 2021
How can I treat symptoms of COVID-19 at home?
Your healthcare provider might recommend the following to relieve symptoms and support your body’s natural defenses:• Taking medications, like acetaminophen or ibuprofen, to reduce fever• Drinking water or receiving intravenous fluids to stay hydrated• Getting plenty of rest to help the body fight the virus
Can I stay at home to recover if I have only mild symptoms of COVID-19?
Most people with COVID-19 have mild illness and can recover at home without medical care. Do not leave your home, except to get medical care. Do not visit public areas.
How long after transfusion does plasma have a lower risk of death?
An updated retrospective analysis of data collected through the EAP indicated that patients who received high-titer plasma had a lower relative risk of death within 30 days after transfusion than patients who received low-titer plasma (relative risk 0.82; 95% CI, 0.67–1.00). 20
When was the EUA issued?
Rationale for Recommendation. On August 23, 2020 , the FDA issued an EUA for convalescent plasma for the treatment of hospitalized patients with COVID-19 based on retrospective, indirect evaluations of efficacy generated from a large Expanded Access Program (EAP). The EAP allowed for the use of convalescent plasma regardless of titer.
Does plasma contain antibodies to SARS?
Plasma from donors who have recovered from COVID -19 may contain antibodies to SARS-CoV-2 that may help suppress the virus and modify the inflammatory response. 1 The Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for convalescent plasma for the treatment of certain hospitalized patients with COVID-19.
What are the side effects of a blood transfusion?
The most common side effects are mild allergic reactions such as hives or itchiness (1-3%). As with all blood products, there is a low risk of transfusion-transmitted infection; however, the odds of contracting viruses such as HIV or Hepatitis C are approximately 1 in 2 million.
What is the EUA authority?
EUA authority allows the FDA to help strengthen the nation’s public health protections by facilitating the availability and use of specific treatments and strategies needed during public health emergencies. In the case of COVID-19, the current EUA allows for the widespread use of convalescent plasma without the need to obtain an emergency IND ...
What is the purpose of convalescent plasma?
Convalescent plasma is the cell-free liquid part of blood (plasma) drawn from people who have recovered (convalesced) from COVID-19. It contains antibodies that help fight the virus off.
Can volunteers donate plasma?
Volunteers who recover from COVID-19 are screened for eligibility to donate. If they are eligible, they give their plasma. It’s very similar to blood donation. The plasma is then given to a sick person. It’s really important that a lot of people donate because doctors can only create one dose from each recovered person.
What is convalescent plasma?
Convalescent means recovering, so this term refers to plasma sourced from people who’ve recovered from COVID-19. When someone gets sick with — and generates antibodies for — the novel coronavirus, those antibodies remain in their plasma (the liquid part of blood) for an unknown period of time.
Is convalescent plasma effective as a treatment for COVID-19?
The existing evidence is minimal, but encouraging. A 20,000-patient Mayo Clinic study conducted in June found that seven-day mortality rates declined to 8.6 percent — down from 12 percent in a previous study of the first 5,000 patients to receive transfusions.
Is convalescent plasma safe?
Like any blood or plasma transfusion, convalescent plasma transfusions can cause allergic reactions, transfusion-associated circulatory overload ( TACO ), and transfusion-associated acute lung injury ( TRALI ).
Has the FDA issued emergency-use authorizations before?
Yes — most recently, the FDA issued emergency-use authorizations (or EUAs) for remdesivir, the anti-viral that appears most effective in severe cases, and for hydroxychloroquine, an anti-malarial frequently touted by President Trump. The FDA withdrew its EUA for hydroxychloroquine in June, highlighting serious side effects.
Can I donate convalescent plasma?
Yes — since April, FDA commissioner Stephen Hahn has encouraged people who have recovered from COVID-19 to donate plasma, and the government has spent $8 million on radio and internet ads encouraging the same. The FDA’s website has more information about how to donate plasma.
What is a convalescent plasma?
Convalescent plasma is a liquid part of the blood that contains antibodies (proteins) to certain infections , such as to COVID-19. Convalescent plasma for COVID-19 is collected from eligible patients who have fully recovered from the virus. Patients who donate must have a documented laboratory-confirmed diagnosis of COVID-19, ...
When did the FDA issue the EUA?
On August 23, 2020 the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for use of convalescent plasma for COVID–19 treatment. According to the FDA, "this product may be effective in treating COVID-19 and that the known and potential benefits of the product outweigh the known and potential risks of the product.".
What is an EUA?
The FDA has now approved an Emergency Use Authorization (EUA) process for convalescent plasma therapy. An EUA can be issued on an investigational agent when the FDA believes a treatment is effective in treating COVID-19 and that the potential benefits of the product outweigh the potential risks. Doctors across the nation may have easier access ...
Is convalescent plasma effective?
The FDA believes that COVID-19 convalescent plasma may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients. However, according to the National Institutes of Health (NIH), there are insufficient data to recommend either for or against the use of COVID-19 convalescent plasma for ...

Why It's Done
- Convalescent plasma therapy may be given to people with COVID-19who are in the hospital and are early in their illness or have a weakened immune system. Convalescent plasma therapy may help people recover from COVID-19. It may lessen the severity or shorten the length of the disea…
Risks
- Blood has been used to treat many other conditions. It's usually very safe. The risk of getting COVID-19from convalescent plasma hasn't been tested yet. But researchers believe that the risk is low because donors have fully recovered from the infection. Convalescent plasma therapy has some risks, such as: 1. Allergic reactions 2. Lung damage and difficulty breathing 3. Infections s…
What You Can Expect
- Your doctor may consider convalescent plasma therapy if you're in the hospital with COVID-19and you are early in your illness or you have a weakened immune system. If you have questions about convalescent plasma therapy, ask your doctor. Your doctor will order convalescent plasma that is compatible with your blood type from your hospital's local blood supplier.
Results
- It's not yet known if convalescent plasma therapy will be an effective treatment for COVID-19. You might not experience any benefit. However, this therapy might help you recover from the disease. Data from several clinical trials, studies and a national access program suggest that convalescent plasma with high antibody levels may lessen the severity or shorten the duration of COVID-19 in …
Clinical Trials
- Explore Mayo Clinic studiesof tests and procedures to help prevent, detect, treat or manage conditions.
Recommendations
- The COVID-19 Treatment Guidelines Panel (the Panel) recommends against the use of COVID-19 convalescent plasma for the treatment of COVID-19 in hospitalized patients without impaired humoral immuni...
- There is insufficient evidence for the Panel to recommend either for or against the use of COVID-19 convalescent plasma for the treatment of COVID-19 in:
Rationale
- For Hospitalized Patients Without Impaired Humoral Immunity
Clinical data on the use of convalescent plasma for the treatment of COVID-19, including data from several randomized trials and the U.S. Expanded Access Program (EAP) for Convalescent Plasma, are summarized in Table 3b. The EUA for convalescent plasma for the treatment of hos… - For Nonhospitalized Patients Without Impaired Humoral Immunity
Current data are insufficient to establish the safety or efficacy of convalescent plasma in nonhospitalized patients with COVID-19. Convalescent plasma is not authorized for nonhospitalized patients with COVID-19 under the EUA. Data from a double-blind, placebo-contr…
Considerations in Pregnancy
- The safety and efficacy of using COVID-19 convalescent plasma during pregnancy have not been evaluated in clinical trials, and published data on its use in pregnant individuals with COVID-19 are limited to case reports.38 Pathogen-specific immunoglobulins (Ig) are used clinically during pregnancy to prevent infection from varicella zoster virus and rabies virus and have been used i…
Considerations in Children
- The safety and efficacy of COVID-19 convalescent plasma have not been systematically evaluated in pediatric patients. Published literature on its use in children is limited to case reports and case series, as well as a systematic review of these reports. A few clinical trials of COVID-19 convalescent plasma in children are ongoing. The use of convalescent plasma may be consider…
Adverse Effects
- Available data suggest that serious adverse reactions following the administration of COVID-19 convalescent plasma are infrequent and consistent with the risks associated with plasma infusions for other indications. These risks include transfusion-transmitted infections (e.g., HIV, hepatitis B, hepatitis C), allergic reactions, anaphylactic reactions, febrile nonhemolytic reaction…
Clinical Trials
- Randomized clinical trials evaluating convalescent plasma for the treatment of COVID-19 are underway. Please see ClinicalTrials.govfor the latest information.