Which monoclonal antibodies are used in systemic lupus erythematosus (SLE)?
A number of monoclonal antibodies (mAb) are now under investigation in clinical trials to assess their potential role in Systemic Lupus Erythematosus (SLE). The most frequently used mAb is rituximab, which is directed against CD20, a membrane protein expressed on B lymphocytes.
What are the clinical trials of systemic lupus erythematosus (SLE)?
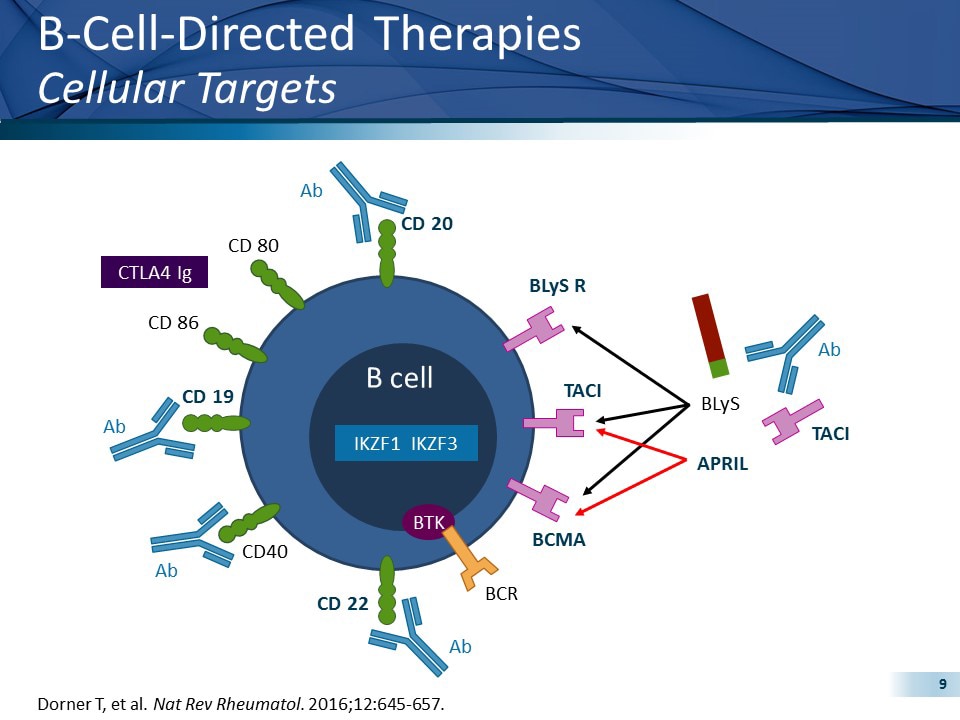
The trials that have been carried out, or are currently under way, include a variety of agents in view of the diversity of the disturbances of the immune system encountered in patients with SLE and are diagrammatically depicted in Figure 1. Open in a separate window Figure 1 Molecules targeted therapeutically in patients with SLE.
What is the role of anifrolumab in systemic lupus erythematosus (SLE)?
Chronic activation of the type I interferon (IFN) pathway plays a critical role in systemic lupus erythematosus (SLE) pathogenesis. Anifrolumab is a human monoclonal antibody to the type I IFN receptor subunit 1, which blocks the action of type I IFNs.
Can we treat systemic lupus erythematosus with B cell therapy?
Systemic lupus erythematosus (SLE) is an astonishing heterogeneous multisystem autoimmune disease with a quite unpredictable outcome. Patients suffering from SLE are typically treated with corticosteroids and immunosuppressive agents (1). An eminent direct or indirect target of novel therapeutic approaches has been the lupus B cell (2–4).

What was the first monoclonal antibody approved?
Orthoclone OKT3The first licenced monoclonal antibody was Orthoclone OKT3 (muromonab-CD3) which was approved in 1986 for use in preventing kidney transplant rejection [7]. It is a monoclonal mouse IgG2a antibody whose cognate antigen is CD3. It works by binding to and blocking the effects of CD3 expressed on T-lymphocytes.
Which is the monoclonal antibody approved for the treatment of systemic lupus erythematosus?
Benlysta is a human monoclonal antibody that was approved for the treatment of lupus by the U.S. Food and Drug Administration (FDA) on March 9, 2011 and for lupus nephritis on December 17, 2020.
What is first line treatment for lupus?
Hydroxychloroquine is first-line treatment unless contraindicated and is useful in almost all manifestations of lupus. Other treatments are titrated against type and severity of organ involvement. Monoclonal antibodies have a limited role in the management of lupus.
Which treatments are recommended for systemic lupus erythematosus?
The medications most commonly used to control lupus include:Nonsteroidal anti-inflammatory drugs (NSAIDs). ... Antimalarial drugs. ... Corticosteroids. ... Immunosuppressants. ... Biologics.
What drugs are approved for SLE?
TreatmentHydroxychloroquine (HCQ): HCQ is an antimalarial agent approved for SLE in 1957. ... Glucocorticoids (GCs): GCs that are often used include methylprednisolone and prednisone. ... Immunosuppressants: Immunosuppressants include azathioprine (AZA), mycophenolate mofetil (MMF), and cyclophosphamide (CYC).More items...•
What are monoclonal antibodies and lupus?
One monoclonal antibody is approved for systemic lupus, called Benlysta (Belimumab). It blocks an immune system protein involved in creating auto-antibodies (antibodies that attack the body's tissue). The new monoclonal antibody works in a different way, Karnell explained.
What is the most common treatment for lupus?
Lupus is mainly treated with medicine. The types of drugs that have been used to treat lupus include NSAIDs, corticosteroids and other immune system suppressing drugs, hydroxychloroquine, and the newest lupus drug, Benlysta.
What is standard therapy for SLE?
Nowadays, antimalarials are the basic treatment for every patient with SLE, whereas glucocorticoids should only be used when acutely indicated. If reduction or tapering of glucocorticoids proves impossible, extended immunosuppression with azathioprine, methotrexate, or mycophenolate mofetil is recommended.
Which antibody is most specific for SLE?
The antinuclear antibody (ANA) test is the most sensitive test for SLE and is therefore the best screening assay for ruling out its presence.
What is Smith antibody?
Abstract. Background: Anti-Smith (Sm) antibody is a highly specific antibody for systemic lupus erythematosus (SLE). Despite the remarkable specificity of anti-Sm antibodies for SLE, the association between anti-Sm antibody level and the clinical manifestation of SLE is still unclear.
What cytokines are used in SLE?
Proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha) and iterleukin-6 (IL-6) play an important role in propagating the inflammatory process responsible for tissue damage. Blocking of these cytokines by mAbs can be also a successful therapy for patients with SLE.
What is SLE autoimmune disease?
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by B cell hyperactivity and defective T-cell function, with production of high titer autoantibodies.
What is the role of anifrolumab in SLE?
Chronic activation of the type I interferon (IFN) pathway plays a critical role in systemic lupus erythematosus (SLE) pathogenesis. Anifrolumab is a human monoclonal antibody to the type I IFN receptor subunit 1, which blocks the action of type I IFNs.
What is the role of IFN in Lupus?
Chronic activation of the type I interferon (IFN) pathway plays a critical role in systemic lupus erythematosus (SLE) pathogenesis. Anifrolumab is a human monoclonal antibody to the type I IFN receptor subunit 1, which blocks the action of type I IFNs. Two phase 3 studies (TULIP-1 and TULIP-2) and a phase 2b study ...
What is anifrolumab used for?
Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials. Chronic activation of the type I interferon (IFN) pathway plays a critical role in systemic lupus erythematosus (SLE) pathogenesis.
What is RC18 in Lupus?
B cells are being targeted directly or indirectly in patients with lupus. RC18 is a recombinant human BLyS receptor antibody fusion protein and it is used in a phase III placebo-controlled study plus standard treatment with primary outcome an SRI response rate at week 52 (59).
What are the B cells in Lupus?
The B cell, as a major component of the adaptive immune system, may mediate autoimmune disease. B cells are not only capable of producing autoantibodies after their differentiation into plasma cells, but they also present autoantigens to T cells and they secrete cytokines. Therefore, B cells represent an established and clear target of treatment approaches; lupus B cells have been targeted either directly via regimens that cause B cell depletion or indirectly via regimens affecting B cell survival, or via inhibiting their antigen-receptor-initiated function.
What is Daratumumab used for?
Daratumumab, a mAb approved for the treatment of multiple myeloma, is an IgG1k mAb directed against CD38 causing depletion of plasma cells. Long-lived plasma cells are residents in niches in the bone marrow or (perhaps more importantly) in inflamed tissue and they do not respond to immunosuppressants, including B-cell-targeting treatments. Two patients with severe manifestations of SLE received daratumumab at a dose of 16 mg/kg of body weight once a week for 4 weeks followed by maintenance treatment with I.V. belimumab ( 18 ). Daratumumab treatment resulted in remarkable clinical outcomes not only of severe manifestations such as lupus nephritis, autoimmune hemolytic anemia and autoimmune thrombocytopenia but also on less severe manifestations such as arthritis, skin rashes, pericarditis, cutaneous vasculitis, alopecia, and mucosal ulcers. Daratumumab treatment was also associated with favorable serologic responses. Importantly, previous therapeutic interventions with a variety of agents such as bortezomib, mycophenolate mofetil, and cyclophosphamide were ineffective. Despite the extremely small number of patients, data are encouraging supporting further evaluation of daratumumab in meaningfully larger numbers of patients with SLE. It is of interest however that the authors did not ascribe their anti-CD38 mAb-mediated clinical effect (s) exclusively to reductions of plasma cell numbers. Other circulating cells also express CD38 and their numbers decreased following daratumumab treatment. Among them are subsets of B cells, plasmacytoid dendritic cells, and a greatly expanded CD38 + T cell subpopulation. Only recently it was shown by Katsuyama et al. that this expanded CD38 + CD8 + T cell subset is responsible for the significantly compromised cytotoxicity encountered in patients with lupus ( 19 ).
What is RC18?
Telitacicept (RC18) is a novel recombinant TACI-Fc (transmembrane activator and calcium modulator and cyclophilin ligand interactor) fusion protein that binds to soluble BLyS and APRIL (A proliferation inducing ligand) prohibi ting thus their biological activities, that go beyond the B cells and affect the plasma cells as well. Therefore, telitacicept inhibits the development and survival of mature B cells and plasma cells without affecting early and memory B cells. In a phase 2b study, patients with a Safety of Estrogen in Lupus Erythematosus National Assessment (SELENA)-SLEDAI score ≥8, consistent with active disease, received telitacicept at doses of 80, 160, and 240 mg or placebo along with standard treatment ( 30 ). The primary endpoint was an SRI-4 at week 48. An SRI-4 was achieved in 71.0, 68.3, and 75.8% of the patients who received the 80, 160, and 240 mg doses, respectively, at week 48 and in 33.9% of the patients who received placebo. The proportion of patients achieving at least a 4-point reduction in their SELENA-SLEDAI scores at week 48 was 75.8, 77.8, and 79.0% of the patients in the telitacicept groups and 50.0% of the patients in the placebo group. Adverse events were recorded in 90.3, 92.1, 93.5, and 82.3% of the patients in the 80, 160, and 240 mg telitacicept and placebo groups, respectively. Adverse events were most commonly reactions at the injection site and infections of the upper respiratory tract. If such promising still early results are confirmed in later stage trials, telitacicept could emerge as a promising, and safe option in the management of active SLE.
What is the treatment for SLE?
Patients suffering from SLE are typically treated with corticosteroids and immunosuppressive agents (1). An eminent direct or indirect target of novel therapeutic approaches has been the lupus B cell (2–4).
Does RTX cause B cell depletion?
B cell depletion following RTX treatment is associated with a sharp homeostatic rise of circulating levels of BLyS. Therefore, treatment at the time when circulating BLyS peaks with belimumab might seem like a rational approach not only to sustain depletion but also to avoid B cell population reconstitution as well. The autoimmune B cell subpopulation might be more sensitive to belimumab-mediated BLyS inhibition. A phase II trial assessed the effect of induction therapy with RTX followed by maintenance therapy with belimumab in 43 patients with recurrent or refractory lupus nephritis ( 29 ). Of these, 21 patients received rituximab, cyclophosphamide and glucocorticoids and subsequently weekly belimumab infusions until week 48 and 22 patients received rituximab and cyclophosphamide without belimumab infusions. Complete renal response was defined as an UPCR <0.5, an eGFR ≥120 ml/min/1.73 m 2, or >80% improvement if eGFR was <120 ml/min/1.73 m 2 at baseline. Partial renal response was defined as >50% improvement of the UPCR at baseline. Total and circulating autoreactive B cells were measured by flow cytometry. Renal response (complete or partial) was achieved in 52% of the patients in the belimumab group and in 41% of the patients that did not receive belimumab ( p = 0.452) at week 48. At least one serious infectious adverse event of grade 3 or higher (according to the National Cancer Institute Common Terminology Criteria for Adverse Events) was noticed in 23% of the patients that did not receive belimumab and in 9.5% of the patients in the belimumab group. Sequential therapy with belimumab was generally safe but it does not seem to improve significantly lupus nephritis. This unfavorable clinical response was in contrast to a good and well-sustained B cell depletion profile in the belimumab group. Moreover, the autoreactive B cells were indeed significantly suppressed, despite the disparity in clinical outcomes.
Is sirolimus a macrolide?
Sirolimus is an immunosuppressive macrolide. It blocks activation of T cells and B cells through mTOR (mammalian target of rapamycin) inhibition, reducing thereby their sensitivity to IL-2. Activation of mTOR plays a role in lupus T cell signaling dysregulation. Such mTOR-mediated lupus T cells defects were described by Fernandez et al. from the Perl Lab ( 46, 47 ). A prospective, open-label, single-arm clinical trial sirolimus was administered in 40 patients with SLE for 12 months ( 48 ). Patients with severe or life-threatening manifestations of SLE, proteinuria (an UPCR higher than 0.5) and hematological abnormalities such as anemia, leukopenia and thrombopenia had been excluded. Eleven patients discontinued the study due to lack of compliance or lack of tolerance. SLEDAI and BILAG scores were decreased in 16 out of 29 patients that completed treatment. Mean SLEDAI score was decreased from 10.2 at enrollment to 4.8 after 12 months of treatment ( p < 0.001) and the mean BILAG score was decreased from 28.4 at enrollment to 17.4 after 12 months of treatment ( p < 0.001). The mean daily dose of prednisone was decreased from 23.7 to 7.2 mg ( p < 0.001) at 12 months after sirolimus initiation. Sirolimus treatment resulted in a raise of the previously reduced CD4 + FoxP3 + regulatory T cells and CD8 + memory T cells. It also decreased the previously increased IL-4 and IL-17 production by CD4 + and CD3 + CD4 − CD8 double-negative T cells after 12 months of treatment. CD8 + memory T cells were selectively increased in patients with clinical improvement. Perhaps more importantly, the depletion of CD62L − CD197 − effector memory CD8 + T cells was reportedly a predictor of a good sirolimus response.