
Randomized controlled trials are the most reliable method available for testing new treatments. They have become the standard that pharmaceutical companies must meet for calculating and proving the level of efficacy and safety of an experimental drug.
What is randomization in clinical trials?
What Are Clinical Trials? Clinical trial randomization is the process of assigning patients by chance to groups that receive different treatments. In the simplest trial design, the investigational group receives the new treatment and the control group receives standard therapy.
What is a randomized controlled trial?
What is a randomized controlled trial? Randomized controlled trials are the most reliable method available for testing new treatments. They have become the standard that pharmaceutical companies must meet for calculating and proving the level of efficacy and safety of an experimental drug.
What are the benefits of randomization in nursing?
INTRODUCTION. The basic benefits of randomization are as follows: it eliminates the selection bias, balances the groups with respect to many known and unknown confounding or prognostic variables, and forms the basis for statistical tests, a basis for an assumption of free statistical test of the equality of treatments.
How do you randomize patients to different interventions?
There are two processes involved in randomizing patients to different interventions. First is choosing a randomization procedure to generate an unpredictable sequence of allocations; this may be a simple random assignment of patients to any of the groups at equal probabilities, may be "restricted", or may be "adaptive."
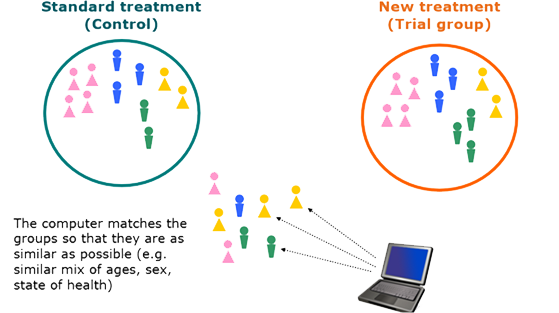
What does randomized mean in research?
(RAN-duh-mih-ZAY-shun) In research, the process by which participants in clinical trials are assigned by chance to separate groups that are given different treatments or other interventions.
What is an example of a randomized study?
Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments.
What is a randomized process?
In the simplest case, randomization is a process by which each participant has the same chance of being assigned to either intervention or control. An example would be the toss of a coin, in which heads indicates intervention group and tails indicates control group.
Why do we Randomise in clinical trials?
Randomized controlled trial is widely accepted as the best design for evaluating the efficacy of a new treatment because of the advantages of randomization (random allocation). Randomization eliminates accidental bias, including selection bias, and provides a base for allowing the use of probability theory.
How do you randomize patients in clinical trials?
The easiest method is simple randomization. If you assign subjects into two groups A and B, you assign subjects to each group purely randomly for every assignment. Even though this is the most basic way, if the total number of samples is small, sample numbers are likely to be assigned unequally.
What is RCT healthcare?
WHAT IS A RANDOMISED CONTROLLED TRIAL? An RCT is a type of study in which participants are randomly assigned to one of two or more clinical interventions.
What is the purpose of randomization?
The main purpose for using randomization in an experiment is to control the lurking variable and establish a cause and effect relationship. Also, by randomizing an experiment the evidence is more supported. Good. The main purpose for using randomization in an experiment is to make sure that the results are accurate.
What are the 3 steps for randomization?
The process of randomising participants into a trial has three different steps: sequence generation, allocation concealment, and implementation (see box 3).
What is the benefit of randomization?
Randomization prevents the skewing or deliberate manipulation of results. Both participants and research scientists can influence results unless the researchers assign participants to groups at random. Scientists refer to this skewing of results as selection bias.
What are the two types of randomized trials?
Depending on the extent of blinding, RCTs can be classified as open, single-blind, double-blind, triple-blind, and quadruple-blind....Contents.1Randomised controlled trials: the basics Questions7From trials to decisions: the basis of evidence based health care Questions6 more rows
Is a randomized clinical trial the same as a randomized controlled trial?
A clinical trial is a randomized controlled trial only when participants are randomly allocated to the group receiving the treatment and a control group. What participants are allocated among groups receiving different treatments the clinical trial is simply called a randomized trial.
What is a randomized controlled trial?
In clinical research, randomized controlled trials (RCTs) are the best way to study the safety and efficacy of new treatments. RCTs are used to answer patient-related questions and are required by governmental regulatory bodies as the basis for approval decisions. Methods.
How to ensure fair comparison between treatments?
To ensure “fair” comparison between the treatments, the different study groups must be truly comparable. This can be achieved by standardization of, for example, the time(s) of intake of the study medication and the methods used to measure clinical parameters, but most important for comparability is randomization of the participants.
How was the Alife study randomized?
They were randomized taking account of the prognostic factors of age (<36 years or ≥ 36 years) and number of miscarriages (2 or ≥3), and because the study was multicentric they were stratified by study center. If patients were allocated to treatment groups by conscious or unconscious selection for prognosis-related characteristics, rather than randomly, this could lead to biased treatment comparison and distorted results (selection bias).
Why are RCTs used in clinical research?
In clinical research RCTs are used to answer patient-related questions, and in the development of new drugs they form the basis for regulatory authorities’ decisions on approval. Alongside meta-analyses, high-quality RCTs with a low risk of systematic error (bias) provide the highest level of evidence (1, 2).
What is the quality of an RCT?
The quality of an RCT depends on an appropriate study question and study design, the prevention of systematic errors, and the use of proper analytical techniques. All of these aspects must be attended to in the planning, conductance, analysis, and reporting of RCTs. RCTs must also meet ethical and legal requirements.
What is clinical research?
Clinical research lays the groundwork for progress in medicine and is an indispensable prerequisite for evidence-based medicine. Randomized controlled clinical trials (RCTs) are the gold standard for ascertaining the efficacy and safety of a treatment. RCTs can demonstrate the superiority of a new treatment over an existing standard treatment or a placebo. In clinical research RCTs are used to answer patient-related questions, and in the development of new drugs they form the basis for regulatory authorities’ decisions on approval. Alongside meta-analyses, high-quality RCTs with a low risk of systematic error (bias) provide the highest level of evidence (1, 2).
Can RCTs be reliable?
RCTs cannot yield reliable data unless they are planned, conducted, analyzed, and reported in ways that are methodologically sound and appropriate to the question being asked. The quality of any RCT must be critically evaluated before its relevance to patient care can be considered.
Why is randomization used in clinical trials?
Randomization as a method of experimental control has been extensively used in human clinical trials and other biological experiments. It prevents the selection bias and insures against the accidental bias. It produces the comparable groups and eliminates the source of bias in treatment assignments. Finally, it permits the use of probability theory ...
What is an overview of randomization techniques?
An overview of randomization techniques: An unbiased assessment of outcome in clinical research
How does stratified randomization work?
The stratified randomization method addresses the need to control and balance the influence of covariates. This method can be used to achieve balance among groups in terms of subjects’ baseline characteristics (covariates). Specific covariates must be identified by the researcher who understands the potential influence each covariate has on the dependent variable. Stratified randomization is achieved by generating a separate block for each combination of covariates, and subjects are assigned to the appropriate block of covariates. After all subjects have been identified and assigned into blocks, simple randomization is performed within each block to assign subjects to one of the groups.
What is randomization based on?
Randomization based on a single sequence of random assignments is known as simple randomization.[3] This technique maintains complete randomness of the assignment of a subject to a particular group. The most common and basic method of simple randomization is flipping a coin. For example, with two treatment groups (control versus treatment), the side of the coin (i.e., heads - control, tails - treatment) determines the assignment of each subject. Other methods include using a shuffled deck of cards (e.g., even - control, odd - treatment) or throwing a dice (e.g., below and equal to 3 - control, over 3 - treatment). A random number table found in a statistics book or computer-generated random numbers can also be used for simple randomization of subjects.
Why is randomization important in a study?
Randomization ensures that each patient has an equal chance of receiving any of the treatments under study, generate comparable intervention groups, which are alike in all the important aspects except for the intervention each groups receives. It also provides a basis for the statistical methods used in analyzing the data. The basic benefits of randomization are as follows: it eliminates the selection bias, balances the groups with respect to many known and unknown confounding or prognostic variables, and forms the basis for statistical tests, a basis for an assumption of free statistical test of the equality of treatments. In general, a randomized experiment is an essential tool for testing the efficacy of the treatment.
How to do randomization?
Generation of a randomization schedule usually includes obtaining the random numbers and assigning random numbers to each subject or treatment conditions. Random numbers can be generated by computers or can come from random number tables found in the most statistical text books. For simple experiments with small number of subjects, randomization can be performed easily by assigning the random numbers from random number tables to the treatment conditions. However, in the large sample size situation or if restricted randomization or stratified randomization to be performed for an experiment or if an unbalanced allocation ratio will be used, it is better to use the computer programming to do the randomization such as SAS, R environment etc.[1–6]
What are the benefits of randomization?
The basic benefits of randomization are as follows: it eliminates the selection bias, balances the groups with respect to many known and unknown confounding or prognostic variables, and forms the basis for statistical tests, a basis for an assumption of free statistical test of the equality of treatments.
What is a randomized controlled trial?
A randomized controlled trial (or randomized control trial; RCT) is a type of scientific experiment (e.g. a clinical trial) or intervention study (as opposed to observational study) that aims to reduce certain sources of bias when testing the effectiveness of new treatments; this is accomplished by randomly allocating subjects to two or more groups, treating them differently, and then comparing them with respect to a measured response. One group—the experimental group—receives the intervention being assessed, while the other—usually called the control group—receives an alternative treatment, such as a placebo or no intervention. The groups are monitored under conditions of the trial design to determine the effectiveness of the experimental intervention, and efficacy is assessed in comparison to the control. There may be more than one treatment group or more than one control group .
When did randomization start?
Randomized experiments first appeared in psychology, where they were introduced by Charles Sanders Peirce and Joseph Jastrow in the 1880s, and in education.
Why are blinded RCTs used?
Blinded RCTs are commonly used to test the efficacy of medical interventions and may additionally provide information about adverse effects, such as drug reactions. A randomized controlled trial can provide compelling evidence that the study treatment causes an effect on human health.
How many RCTs were there in 2004?
By the late 20th century, RCTs were recognized as the standard method for "rational therapeutics" in medicine. As of 2004, more than 150,000 RCTs were in the Cochrane Library.
What is permuted block randomization?
Permuted-block randomization or blocked randomization: a "block size" and "allocation ratio" (number of subjects in one group versus the other group) are specified, and subjects are allocated randomly within each block. For example, a block size of 6 and an allocation ratio of 2:1 would lead to random assignment of 4 subjects to one group and 2 to the other. This type of randomization can be combined with " stratified randomization ", for example by center in a multicenter trial, to "ensure good balance of participant characteristics in each group." A special case of permuted-block randomization is random allocation, in which the entire sample is treated as one block. The major disadvantage of permuted-block randomization is that even if the block sizes are large and randomly varied, the procedure can lead to selection bias. Another disadvantage is that "proper" analysis of data from permuted-block-randomized RCTs requires stratification by blocks.
How many randomized experiments were published in criminology?
Criminology. A 2005 review found 83 randomized experiments in criminology published in 1982–2004, compared with only 35 published in 1957–1981. The authors classified the studies they found into five categories: "policing", "prevention", "corrections", "court", and "community".
What does it mean to be blinded in an experiment?
The trial may be blinded, meaning that information which may influence the participants is withheld until after the experiment is complete. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators.
RCTs - Comparing Treatment
one (or more) experimental group (s) who receive a new treatment, and a control group, who receive the current standard treatment (which might be the best existing treatment, no treatment or a placebo).
Randomisation
For example, if a doctor chose which treatment a patient should receive as part of a trial, she or he might give the new treatment to sicker patients, or to younger patients. This would make the results of a trial unreliable, as it could exaggerate or hide the effects of the treatment.
Why is randomization important in clinical trials?
Randomization of treatment in clinical trials pose ethical problems. In some cases, randomization reduces the therapeutic options for both physician and patient, and so randomization requires clinical equipoise regarding the treatments.
What is a randomized experiment?
In science, randomized experiments are the experiments that allow the greatest reliability and validity of statistical estimates of treatment effects. Randomization-based inference is especially important in experimental design and in survey sampling .
What is the simplest design for comparing treatments?
In the design of experiments, the simplest design for comparing treatments is the "completely randomized design".
What is randomization in statistics?
In the statistical theory of design of experiments, randomization involves randomly allocating the experimental units across the treatment groups. For example, if an experiment compares a new drug against a standard drug, then the patients should be allocated to either the new drug or to the standard drug control using randomization.
What is the Rubin Causal Model?
The Rubin Causal Model provides a common way to describe a randomized experiment. While the Rubin Causal Model provides a framework for defining the causal parameters (i.e., the effects of a randomized treatment on an outcome), the analysis of experiments can take a number of forms. Most commonly, randomized experiments are analyzed using ANOVA, student's t-test, regression analysis, or a similar statistical test .
What is a restriction on randomization?
Some "restriction on randomization" can occur with blocking and experiments that have hard-to-change factors; additional restrictions on randomization can occur when a full randomization is infeasible or when it is desirable to reduce the variance of estimators of selected effects.
When were randomized experiments invented?
Randomized experiments were institutionalized in psychology and education in the late eighteen-hundreds, following the invention of randomized experiments by C. S. Peirce. Outside of psychology and education, randomized experiments were popularized by R.A. Fisher in his book Statistical Methods for Research Workers, which also introduced additional principles of experimental design.
What is the process of randomization?
Randomization is the process where clinical researchers randomly select people to participate in clinical studies. Randomization can also mean the random application of the treatment tested. The method is based on chance whereby volunteers are assigned to study intervention. The scientific and medical population will regard a clinical study valid and useful when there is proper randomization.
Who determines the randomization procedure?
The biostatisticians and investigators determine the randomization procedure. It should be detailed in the protocol and consider the following:
What is clinical trial?
A clinical trial is a type of research or experiment in clinical research that evaluates a surgical, medical, or behavioral intervention. The study is people-based– for example, it can determine whether a certain vaccine or a new drug is effective and safe. Clinical researchers need to ensure the study results they get are accurate and applicable, so they often use a randomization method to ensure the study results from research are not biased and apply to the medical and scientific population. Here’s what to know about this process and what it means.
Do you have to read informed consent before a study?
It’s mandatory to read and review the informed consent form and know more about the study before the process starts. Upon agreement of the terms and conditions, you’ll get a physical screening. After the confirmation of the screening, you will be ready to participate in the study.
Do placebo groups receive treatment?
When it comes to random allocation, each participant is given a different level of treatment. One group, called the placebo group, does not receive treatment. This is helpful in determining the variations between the two groups.
