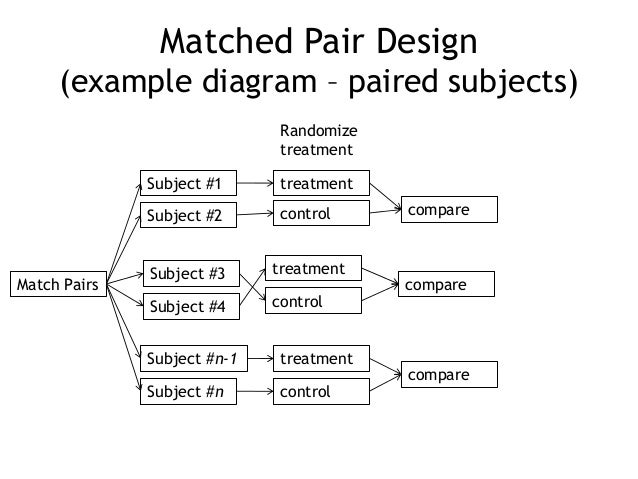
Full Answer
How many subjects should be selected for randomization?
6)These references recommend 200 or more subjects, but it is not possible to determine the exact number. 7)Random allocation rule, truncated binomial randomization, Hadamard randomization, and the maximal procedure are forced balance randomization methods within blocks, and one of them is applied to the block.
How does randomization affect the outcome of clinical trials?
Schul and Grimes stated that trials with inadequate or unclear randomization tended to overestimate treatment effects up to 40% compared with those that used proper randomization. The outcome of the research can be negatively influenced by this inadequate randomization.
What is the difference between as treated and as randomized analysis?
ITT analysis is referred as ‘as randomized’ - the opposite of the term ‘as treated’. In majority of cases, if all randomized subjects receive their allocated treatments and if there is no randomization error, the ‘as treated’ analysis will be the same as 'as randomized' analysis.
What is the best way to randomize a study?
Other methods include using a shuffled deck of cards (e.g., even - control, odd - treatment) or throwing a dice (e.g., below and equal to 3 - control, over 3 - treatment). A random number table found in a statistics book or computer-generated random numbers can also be used for simple randomization of subjects.
How do you determine if an experiment is randomized?
Experimental designs, also called randomized experiments, are characterized by two distinguishing features: (i) the conscious manipulation by the researcher of a treatment or, more generically, an independent variable of interest, and (ii) the random assignment of units to treatment and control groups (Fig. 4).
What does it mean when subject is randomized?
Randomization is a method of allocating subjects in a clinical trial to treatment groups such that every subject has an equal chance of receiving any one of the treatments or interventions. This can be achieved by any fair method that assigns subjects in a completely unpredictable fashion.
What makes a study randomized?
Definition. A study design that randomly assigns participants into an experimental group or a control group. As the study is conducted, the only expected difference between the control and experimental groups in a randomized controlled trial (RCT) is the outcome variable being studied.
How do you randomize a subject in a study?
The easiest method is simple randomization. If you assign subjects into two groups A and B, you assign subjects to each group purely randomly for every assignment. Even though this is the most basic way, if the total number of samples is small, sample numbers are likely to be assigned unequally.
How do you randomize a clinical study?
The most common and basic method of simple randomization is flipping a coin. For example, with two treatment groups (control versus treatment), the side of the coin (i.e., heads - control, tails - treatment) determines the assignment of each subject.
When does randomization occur in clinical trials?
At several points during and at the end of the clinical trial, researchers compare the groups to see which treatment is more effective or has fewer side effects. Randomization helps prevent bias. Bias occurs when a trial's results are affected by human choices or other factors not related to the treatment being tested.
What is an example of a randomized clinical trial?
An active-controlled randomized trial might compare diabetic patients with implanted insulin pumps against diabetic patients who receive multiple insulin injections (the control group). Randomization avoids bias by eliminating baseline differences in risk between treatment and control groups.
What is non randomized study?
A clinical trial in which the participants are not assigned by chance to different treatment groups. Participants may choose which group they want to be in, or they may be assigned to the groups by the researchers.
What are the key components of randomized control trials?
An RCT's essential elements are randomization, preordained outcome measures, and blinding.
How do you ensure randomization?
There are various ways to ensure randomization: choosing numbers from a container (unrestricted or simple random sampling); restricted randomization where a participant is allocated to a treatment group, while maintaining a balance across treatment groups (permuted block randomization); and where participants are ...
How do you randomly assign treatments in statistics?
For example, you might use simple random sampling, where participants names are drawn randomly from a pool where everyone has an even probability of being chosen. You can also assign treatments randomly to participants, by assigning random numbers from a random number table.
What are the 3 steps for randomization?
The process of randomising participants into a trial has three different steps: sequence generation, allocation concealment, and implementation (see box 3).
Why is randomization important in a controlled trial?
Randomized controlled trial is widely accepted as the best design for evaluating the efficacy of a new treatment because of the advantages of randomization (random allocation). Randomization eliminates accidental bias, including selection bias, and provides a base for allowing the use of probability theory.
How does randomization affect evidence-based research?
Randomization plays a crucial role in increasing the quality of evidence-based studies by minimizing the selection bias that could affect the outcomes. In general, randomization places programming for random number generation, random allocation concealment for security, and a separate random code manager.
Why is selection bias inevitable?
Here, a selection bias is inevitable because a researcher or subject can easily predict the allocation of the group. For a small number of subjects, their number in the treatment groups will not remain the same as the study progresses, and the statistical analysis may show the problem of poor power.
Why is randomization used in clinical trials?
Randomization as a method of experimental control has been extensively used in human clinical trials and other biological experiments. It prevents the selection bias and insures against the accidental bias. It produces the comparable groups and eliminates the source of bias in treatment assignments. Finally, it permits the use of probability theory ...
What are the benefits of randomization?
The basic benefits of randomization are as follows: it eliminates the selection bias, balances the groups with respect to many known and unknown confounding or prognostic variables, and forms the basis for statistical tests, a basis for an assumption of free statistical test of the equality of treatments.
Why is random assignment important?
In such instances, random assignment is necessary and guarantees validity for statistical tests of significance that are used to compare treatments. TYPES OF RANDOMIZATION. Many procedures have been proposed for the random assignment of participants to treatment groups in clinical trials.
Where do random numbers come from?
Random numbers can be generated by computers or can come from random number tables found in the most statistical text books . For simple experiments with small number of subjects, randomization can be performed easily by assigning the random numbers from random number tables to the treatment conditions.
Is age a confounding variable in clinical research?
It is well known that the age of the subject affects the rate of prognosis. Thus, age could be a confounding variable and influence the outcome of the clinical research.
Do subjects in different groups differ in any systematic way?
First, subjects in various groups should not differ in any systematic way. In a clinical research, if treatment groups are systematically different, research results will be biased. Suppose that subjects are assigned to control and treatment groups in a study examining the efficacy of a surgical intervention.
Is randomization case sensitive?
The randomization plan is not affected by the order in which the treatments are entered or the particular boxes left blank if not all are needed. The program begins by sorting treatment names internally. The sorting is case sensitive, however, so the same capitalization should be used when recreating an earlier plan.
What is Randomization?
It is a method based on chance by which study participants are assigned to a treatment group. SMART-TRIAL uses randomized blocks with varied random block sizes. Block randomization works by randomizing participants within blocks so that there is an equal number of participants in each group.
Why should I use this feature in my Clinical Study?
Block randomization has become a commonly used technique in clinical trial design to reduce bias. Additionally, it creates balance in the allocation of participants to groups, especially when the sample size is small. It is simple to use, setup and it fulfills all the quality requirements that are relevant to this particular function.
How does Randomization work in SMART-TRIAL?
To use this function you need to follow 3-4 simple steps. 1. You can simply specify that you want to create a clinical study using randomized functionality. 2. You need to specify the total number of subjects that you would like to recruit. 3. And finally, you indicate the names of the groups.
Why do researchers prefer simplified random sampling over stratified randomization?
When the population can’t be categorised into subcategories because of too many differences, or lack of information about the population , then researchers or investigators prefer simplified random sampling over stratified randomization sampling. Stratified randomization is a subcategory of stratified sampling.
What is random sampling?
A simple random sampling involves a sample of subjects from the population. In this, the subjects are chosen randomly from the existing population and are selected for the sample. This process of selecting random subjects for research or analysis is a fair representation of the population. However, when it comes to the samples of a widely varied population, stratified randomization sampling is preferable.
What is stratified randomization?
Stratified Randomization is an essential branch of data science. Industries and businesses are finding the application of data science increasingly useful. Therefore, a lot of beginners, as well as professionals are seeking certification, diploma, degree, or even a doctorate in data science.
What is the process of segmenting potential patient groups into subgroups?
It can be age, gender, ethnicity, medical history, or any other demographic parameter. Patient stratification is the process of segmenting potential patient groups into subgroups, also known as strata, or blocks. Each stratum represents a section of the potential patient population.
What are the complications of clinical trials?
Complications in Clinical Trial. No one has the resources to test a vaccine or drug or treatment on the entire human population, and therefore clinical tests and trials are performed on a limited set of the population that reflects the potential population for the drug in question.
Why is finding the right pool of test subjects important?
Finding and enrolling the right pool of test subjects is one of the most important factors in the world of drug testing and development. Using the right search tool for accurate genetic data helps to refine the data further. It is a common problem faced by researchers when performing patient stratification.
Why is patient stratification important?
The importance of patient stratification is clearly valued in clinical trials. It is the practice of categorising people and results by a parameter other than the provided treatment. It is used to confirm the unbiased allocation of subgroups of humans to trial or investigation.
How is randomization used in medicine?
Randomization is a method whereby subjects are allocated to one of the two risk factor groups by a random mechanism which assures that each individual has an equal chance of being assigned to either group. Tossing a fair coin and allocating an individual to one group or the other based on-the appearance of "heads" or the use of a table of random numbers are examples of such processes. For instance, in studying the efficacy of a new medication relative to a standard one, the names of the patients could be entered sequentially, line by line, in a book, and a number from a table of random numbers could be assigned to each line. The patients assigned even numbers would be allocated to the new medi- cation, those with odd numbers to a standard one. A more sophisticated ran- domized design should be used if we require equal numbers of patients in the two medication groups. After the randomization process has determined that a particular subject should be assigned to a particular group, the investigator must have enough control to implement that assignment. There is clearly no way to conduct a randomized study if the investigator must accept the assignment of people to treatment or comparison groups as determined by nature or by some institutional process (some examples will be given in Sections 4.4 and 4.5). The primary virtue of randomization is that with high probability the two groups will be similar. Indeed, the only initial systematic difference betweed the two groups will be that one received the treatment and the other did not. Therefore, if the treatment has no effect, the distribution of the outcome variable in the two groups would be quite similar. In the next section we provide a more extensive discussion of the properties of randomization.
What is the period between the exposure to the risk factor and the outcome?
Up to now we have presented two types of studies which are generally longi- tudinal; that is, there is a period between the exposure to the risk factor and the outcome which is the period needed by the risk factor to have an effect, if it has any. However, the length of this period may not be known; the outcome may be undetected for a while or the exposure to the treatment may expand over many years. For these various reasons, cross-sectional studies are sometimes done in which the risk and outcome factors are ascertained at the same time. For example, to study the relationship between obesity and heart disease, we might collect data that classified people as obese or nonobese in 1978 and with or
What does "as treated" mean in medical terminology?
The term “as treated” means that when we do analysis/summaries, the treatment assignment is based on the actual treatment the patients receive, not the treatment the patients are supposed to receive.
What is intention to treat?
Typically, ‘Intention-to-Treat’ population can be simply defined as all patients who are randomized. However, the formal definition of the “Intention-to-Treat” population contains several other concepts: The formal definition of the "Intention to Treat" is usually referred to the one suggested by Fisher, LD et al.
When we design a non-inferiority trial to support the product registration, the regulatory agencies may suggest
When we design a non-inferiority trial to support the product registration, the regulatory agencies may suggest the statistical analysis using ‘as treated’ population. In FDA’s Guidance for Industry Non-Inferiority Clinical Trials, it suggested the ‘as-treated’ analysis for primary efficacy endpoint:
Confounding
Many factors can influence whether or not a subject will develop an outcome of interest. As a simple example, consider a study with the goal of determining whether physical activity reduces the risk of heart disease.
Methods of Assignment
The distinguishing feature of an intervention study is that the investigators assign subjects to a treatment (or "exposure") in order to establish actively treated groups of subjects and a comparison group.
