
Biological Nitrification Process in Waste Water Treatment System
- Definition. The removal of nitrogen by biological nitrification and denitrification is a two-step process. In the first...
- Purpose of Nitrification.
- Nitrification Process. In case of toxic and inhibitory substances in wastewater, two‐sludge suspended growth system may...
Full Answer
What are the steps in the process of nitrification?
Some are adapted to extremes such as very hot or very cold conditions, others to high pressure, and a few, such as Deinococcus radiodurans, to high radiation environments. Microorganisms also make up the microbiota found in and on all multicellular organisms.
What is filtration process in water treatment?
What are the different types?
- Strainers & Straining. Straining is a very simple method in which water is poured through a piece of cloth, and can remove some of the suspended silt and solids, destroying ...
- Gravity. Gravity filtration is a method of filtering impurities by using gravity to pull liquid through a filter.
- Membrane. ...
- Media. ...
- Pressure. ...
What is biotower in waste water treatment?
- Overall machineries and equipment operation - to list down and create all machineries and equipment operation
- Guidance to Operators - to create what are their jobs and routines, and to include the checklist on the frequency
- Guidance to Supervisors (engineer level) - supervisors are actually look int
What is the process of nitrification?
Nitrification is an aerobic process. This is accomplished with the help of certain microorganisms. Nitrification is a biological process mainly carried out by certain autotrophic nitrifying bacterias and involves oxidation of nitrogen compounds, main ammonia to nitrite and nitrate, that can be used by living organisms.
Which bacteria are responsible for nitrification?
Aerobic autotrophic bacteria are responsible for nitrification in activated sludge and biofilm processes; Two‐step process in nitrication involve two groups of bacteria; First stage, ammonia is oxidized to nitrite by one group (Nitrosomonas) and second stage, nitrite is oxidized to nitrate by another group of autotrophic bacteria (Nitrobacter) ...
What is the process of removing nitrogen?
Definition. The removal of nitrogen by biological nitrification and denitrification is a two-step process. In the first step (nitrification), ammonia is converted aerobically to nitrate (NO 3− ). In the second step (denitrification), nitrates are converted to N 2 O or nitrogen gas (N 2) under anoxic conditions.
What bacteria are oxidized to nitrate?
Other autotrophic bacteria for oxidation of nitrite to nitrate (prefix with Nitro‐): Nitrococcus, Nitrospira, Nitrospina, and Nitroeystis.
Is nitrification inhibited by ammonia?
Nitrification is also inhibited by un‐ionized ammonia (NH 3) or free ammonia, and un‐ionized nitrous acid (HNO 2 ); Inhibition effects are dependent on total nitrogen species concentration, temperature, and pH. Let us know in the comments what you think about the concepts in this article!
What is the process of nitrification?
Nitrification is a biological process mainly carried out by certain autotrophic nitrifying bacterias and involves oxidation of nitrogen compounds, mainly ammonia to nitrite and nitrate , that can be used by living organisms. Nitrification occurs in mainly two steps:
What is meant by nitrification and denitrification?
What is meant by nitrification and denitrification?#N#Ans: Nitrification is a process where ammonia is converted into nitrite and followed by further oxidation of nitrite to nitrate with the help of nitrifying bacteria. Whereas in denitrification a few microorganisms such as Pseudomonas convert nitrates into nitrogen or into some oxides.
Why is the nitrogen cycle important?
This is because it maintains the total amount of nitrogen present in the environment, soil and water. Nitrogen is part of amino acids and proteins, which are the building blocks of life. Atmospheric nitrogen needs to be converted into its usable forms through nitrification, ammonification and denitrification regularly to maintain life on the planet.
Why is nitrification important?
3. Nitrification plays an important role in agricultural fields to increase the yield of essential crops like rice, wheat and many leguminous plants. 4. Researchers in biotechnology are attempting to transfer nif genes from microorganisms like Pseudomonas to crop plants to get a better yield of crops.
What is the role of nitrogen in living things?
Nitrogen is an important constituent of protein, DNA, RNA, and enzymes. Since elemental or pure nitrogen cannot be directly utilized by most living organism s. 2. Nitrification is the process by which nitrogen becomes available for plants. 3.
What fungi can convert ammonia into nitrate?
Aspergillus flavus, Penicillium are some common fungi, which can also convert ammonia into nitrite. The oxidation of nitrite to nitrate is an essential step as nitrate is the chemical form of nitrogen used by most plants from soil or water.
What is the name of the process of forming nitrate from nitrogen?
1. Ammonification: The formation of Ammonia from nitrogen is called Ammonification. 2. Nitrification: The formation of Nitrate from Ammonia with the help of specialized microorganisms is called Nitrification.
What is nitrification in wastewater?
Nitrification is one of the two primary mechanisms for ammonia removal in aerobic wastewater systems. Nitrification is a two-step process performed by two categories of bacteria, ammonia oxidizers and nitrite oxidizers. Ammonia oxidizing bacteria (AOB) convert ammonia to nitrite through the ammonia oxidation pathway.
What is the primary nitrifying bacteria in wastewater?
Traditionally Nitrosomonas (AOB) and Nitrobacter (NOB) have been considered the primary nitrifying bacteria present in wastewater systems, but there is increasing evidence that this is not necessarily the case. Different nitrifying bacteria tend to predominate to different conditions.
How long do nitrifiers stay in the refrigerator?
Testing, in this case, was run on nitrifiers that were stored in the refrigerator for approximately 6 months after growth in order to determine if nitrifiers remain active after this period. Nitrifier reactors were run in triplicate, while control reactors (no nitrifiers) were run in replicate.
Why are nitrifiers needed?
Nitrifiers require specific operating conditions to function effectively and are more sensitive to toxicity and other adverse conditions than most wastewater heterotrophs. If optimal conditions are present in a wastewater plant, nitrifier populations will be more robust and less susceptible to upsets.
What temperature do nitrifiers need to grow?
Nitrifiers prefer a temperature range between 15–30°C. Nitrifiers have trouble growing fast enough to maintain a population below 15°C, and sometimes have problems with low dissolved oxygen levels above 30°C as oxygen solubility decreases in higher temperatures. Nitrifiers have been recorded functioning effectively outside this temperature range as the bacterial population is often able to acclimate to varied temperatures. Rapid temperature changes also have significant adverse effects on nitrification since nitrifiers are unable to adjust quickly due to their slow growth rates. Nitrifiers have been observed functioning over 35°C (as high as 52°C) in a recent study (Knight, 2019), which had been thought to be near impossible.
What is the primary form of ammonia removal?
Nitrification becomes the primary form of ammonia removal after levels of BOD have been exhausted. Ammonia is often released in the decomposition of urea but will also appear in lower quantities due to the degradation of proteins and other nitrogen-containing molecules. Organic forms of nitrogen, such as amino acids and proteins, tend to be favored forms of nitrogen for heterotrophic bacteria. This means in wastewater treatment systems some excess ammonia is often present that must be oxidized by nitrifiers to achieve effluent limits for ammonia. In cases of lost nitrification , this extra ammonia is what is observed in waste discharge.
Why is alkalinity important for nitrification?
The first is nitrifiers use dissolved carbon dioxide, carbonate, and bicarbonate as their carbon source for autotrophic production of glucose, and (CO2, HCO3-, CO3-2) are major contribu tors to a system’s alkalinity. The second reason is nitrifiers produce nitric acid during ammonia oxidation and if alkalinity is low, pH fluctuations due to acid production can lead to poor nitrifier growth. This means in cases of low alkalinity, it is much more beneficial to supplement alkalinity with carbonate or bicarbonate, rather than other commonly added basic compounds such as sodium hydroxide or magnesium hydroxide. Typically, an alkalinity around 100 ppm is optimal for nitrifier function but there is no harm in being lower or higher assuming the system is maintaining a stable pH and nitrification is functioning well enough to handle the rate of ammonia loading in a system. 8.64 mg/L bicarbonate (HCO3) is considered adequate to remove 1 ppm of ammonia in wastewater systems based on a model of nitrification from 1976 (USEPA, 2002).
What is biological nitrification?
Biological nitrification is the microbe-mediated process of oxidizing ammonia to remove nitrogenous compounds from wastewaters. Domestic sewage typically contains 20 to 40 mg/L of ammonia nitrogen (NH 4- N). Organic matter containing nitrogen, e.g., protein and nucleic acid, also biodegrades to release ammonia.
What is the main cause of nitrification?
Excess nitrogen in the form of ammonia in finished water can be the principal cause of nitrification since ammonia serves as the primary substrate in the nitrificaiton process. Ammonia, nitrate and nitrite can typically be found in surface water supplies as a result of natural processes.
What is the process of oxidizing ammonia to nitrate?
As shown in the nitrification process , ammonia is first oxidized to nitrite ions, then the nitrite ions are oxidized to nitrate ions. Each oxidation is carried out by a different group of bacteria, the ammonia oxidizing bacteria (AOB) and the nitrite oxidizing bacteria (NOB).
Why is pH important in nitrification?
First, a reduction of total alkalinity may accompany nitrification because a significant amount of bicarbonate is consumed in the conversion of ammonia to nitrite. A model that was developed in 1974 indicates that 8.64 mg/L of bicarbonate (HCO 3) will be utilized for each mg/L of ammonia-nitrogen oxidized. While reduction in alkalinty does not impose a direct public health impact, reductions in alkalinity can cause reductions in buffering capacity, which can impact pH stability and corrosivity of the water toward lead and copper. Secondly, nitrifying bacteria are very sensitive to pH. Nitrosomonas has an optimal pH between approximately 7.0 and 8.0, and the optimum pH range for Nitrobacter is approximately 7.5 to 8.0. Some utilities have reported that an increase in pH (to greater than 9) can be used to reduce the occurrence of nitrification.
What are the operations of nitrifying bacteria?
Operational practices that ensure short residence time and circulation within the system can minimize nitrification problems.
How does ammonia stripping work?
Ammonia stripping is the removal of nitrogen from wastewater when the nitrogen is in gaseous ammonia form . Ammonia is a volatile substance, which means that is has a tendency to leave the wastewater and enter the atmosphere. Ammonia (NH 3) and ammonium (NH 4) exist in equilibrium with each other based on the pH. Most of the ammonia-nitrogen in municipal wastewater is in the ammonium form because of its neutral pH range (between 6 and 8). Therefore, chemicals such as lime or sodium hydroxide must be added to raise the pH to the 10.5 to 11.5 range. This will effectively "convert" the ammonium in the wastewater to ammonia. The stripping effect is achieved by introducing the high pH wastewater into th etop of a tower packed with fixed media (or "packing"). Air is blown into the bottom of the tower and flows in a countercurrent fashion with the incoming wastewater. The intimate contact between wastewater droplets and fresh air encourages the ammonia to volatilize from the wastewater to the exiting air stream.
How do bacteria remove nitrogen from wastewater?
Bacteria remove nitrogen from wastewater by a two step biological processes: nitrification followed by denitrification. Technically, it is a three step process: ammonification precedes nitrification and denitrification.
What is biological nitrification?
Biological nitrification is the microbe-mediated process of oxidizing ammonia to remove nitrogenous compounds from wastewaters. Domestic sewage typically contains 20 to 40 mg/L (ppm) of ammonia nitrogen (NH4-N). Organic matter containing nitrogen, e.g., protein and nucleic acid, also biodegrades to release ammonia. Releasing this ammonia into receiving streams has a direct toxic effect on fish and other animals and, in addition, causes significant oxygen depletion as illustrated in the following equation.
What is the process of oxidizing ammonia?
As shown in the nitrification process equations, ammonia is first oxidized to nitrite ions, then the nitrite ions are oxidized to nitrate ions. Each oxidation is carried out by a different group of bacteria, the ammonia oxidizing bacteria (AOB) and the nitrite oxidizing bacteria (NOB). Each group of bacteria has multiple species and a wastewater treatment process may contain several species of each group. In fact, the process may also include Archaea which are distinct from the bacteria but function similarly in many cases.
What is the first step of nitrification?
In the first step of nitrification, ammonia-oxidizing bacteria oxidize ammonia to nitrite according to below equation: NH3 + O2 → NO2– + 3H++ 2e–. Nitrosomonas is the most frequently identified genus associated with this step, although other genera, including Nitrosococcus and Nitrosospira, may be involved.
What is the process of removing nitrates from water?
Denitrification is removal of Nitrates from water or waste water. There are two basic approaches for Denitrification . Nitrate is a stable and highly soluble ion with a low potential for precipitation or adsorption. These properties make it difficult to remove from water using treatment processes such as filtration or activated carbon adsorption.
What is the process of removing ammonia from domestic wastewater?
Untreated domestic wastewater contains ammonia. Nitrification is a biological process that converts ammonia to nitrite and then to nitrate. If standards require that the resulting nitrate be removed, one treatment alternative is the process of denitrification , in which nitrate is reduced to nitrogen gas.
What is the process of oxidizing ammonia into nitrate?
Nitrification is a microbial process by which ammonia is sequentially oxidized to nitrite and then to nitrate. The nitrification process is accomplished primarily by two groups of autotrophic nitrifying bacteria that can build organic molecules by using energy obtained from inorganic sources––in this case, ammonia or nitrite.
Why is methanol used in water distribution?
Methanol is normally used as carbon source to facilitate the biological denitrification process. The major advantage of this system is it does not alter the composition of the water except removing the Nitrates.
What is the process of ion migration?
In the electrodialysis (ED) process, ions migrate through ion-selective semi permeable membrane as a result of electrically charged membrane surfaces. A positive electrode (cathode) and a negative electrode (anode) are used to charge the membrane surfaces and attract oppositely charged ions. As a result of this process, ions such as nitrate are removed from the raw water. In electrodialysis reversal (EDR), the charge on the membranes is periodically reversed to minimize scale development. An electrodialysis reversal package plant is depicted below.
Why is oxygen concentration minimized in denitrification?
Oxygen is typically is minimized by avoiding aeration of the waste water and having a high concentration of BOD so that microorganisms use all the oxygen. In waste water treatment facilities the BOD of waste water is reduced in ...
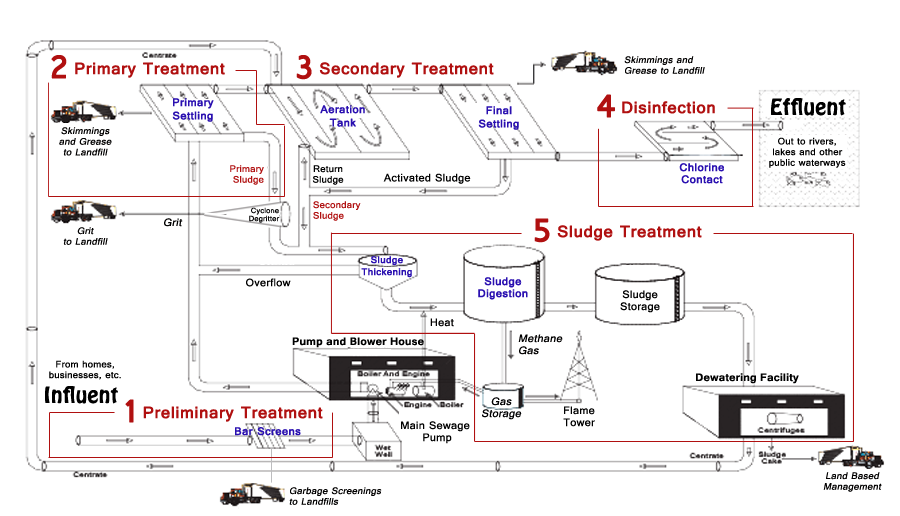
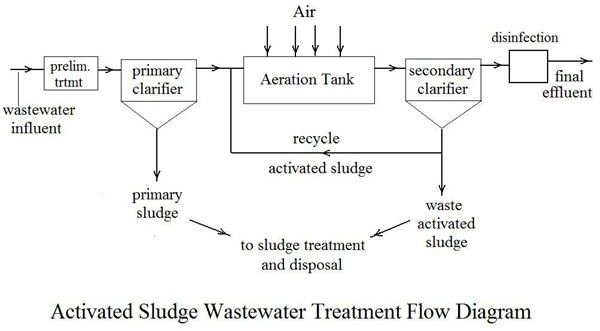
Definition
Purpose of Nitrification
- Effect of ammonia on receiving water with respect to DO concentrations and fish toxicity
- Need to provide nitrogen removal to control eutrophication
- Need to provide nitrogen control for water‐reuse applications including groundwater recharge
- Drinking water maximum MCL for nitrate nitrogen is 45 mg/L as nitrate or 10 mg/L as nitrogen
Nitrification Process
- Nitrification process in waste water treatment is accomplished in both suspended growth and attached growth biological processes
Factors Affecting Process of Nitrification
- Environmental Factors: pH
1. Nitrification process in waste water treatment is pH sensitive and rates decline significantly at pH values below 6.8; Optimal nitrification rates occur at pH values in 7.5‐8.0 range; pH of 7.0 to 7.2 is normally used; 2. Low alkaline waters require alkalinity to be added to maintain acceptabl… - Environmental Factors: Toxicity
1. Nitrifiers are good indicators of presence of organic toxic compounds at low concentrations; 2. Toxic compounds include: Solvent organic chemicals, amines, proteins, tannins, phenolic compounds, alcohols, cyanates, ethers, carbamates, and benzene
Reactions Involved in Nitrification Process
Conversion of Ammonia to Nitrite
- This step is carried forward by ammonia-oxidizing bacteria, also known as nitrifying bacteria, that oxidize ammonia to nitrite. \({\rm{N}}{{\rm{H}}_{\rm{3}}}{\rm{ + }}{{\rm{O}}_{\rm{2}}} \to {\rm{N}}{{\rm{O}}_{{{\rm{2}}^{\rm{ – }}}}}{\rm{ + 3}}{{\rm{H}}^{\rm{ + }}}{\rm{ + 2}}{{\rm{e}}^ – }\) During this reaction, ammonia reacts with intermediate hydroxylamine in the presence of two en…
Oxidation of Nitrate from Nitrite
- The nitrite that is formed is oxidised to nitrate with the help of bacterias, mostly Nitrobacter, during this second step. Below is the chemical equation for the reaction that takes place here: \({\rm{NO}}_{\rm{2}}^{\rm{ – }}{\rm{ + }}{{\rm{H}}_{\rm{2}}}{\rm{O}} \to {\rm{NO}}_{\rm{3}}^{\rm{ – }}{\rm{ + 2}}{{\rm{H}}^{\rm{ + }}}{\rm{ + 2}}{{\rm{e}}^ – }\) Few other genera of bacteria also he…
Process of Nitrification in The Nitrogen Cycle
- Nitrogen in the air is mostly present in its elemental form that is chemically inert and cannot be used by the majority of organisms. Hence, it needs to be converted to forms that can be used by plants from soil or water. This conversion is mainly carried out by certain nitrogen-fixing bacteria such as Azotobacter (freely in soil), Rhizobium (found in root nodules of leguminous plants), etc…
Microorganisms Involved in Nitrogen Fixation
- Bacterias play an important role in natural or industrial nitrogen fixation. Nitrogen cycle depends upon at least four different kinds of bacteria known as the decay causer, nitrifiers, denitrifiers and lastly, nitrogen fixers. Microbes, like bacteria, fungus, actinomycetes, cyanobacteria help in the conversion of nitrogen and formation of ammonia. This can be categorised like: 1. Non-symbioti…
Factors Affecting Nitrification Process
- Though nitrification is a natural process that occurs regularly in ecosystems, in order to increase plant yield, it is also induced artificially. Factors that lead to a decrease in the rate of nitrification are called nitrification inhibitors. Below are five factors that may influence the rate of nitrification: 1. Water Content in Soil: Increase or decrease in water content reduces the nitrogen fixation in s…
Functions of Nitrification
- Below are some functions of the nitrification process: 1. Nitrogen is an important constituent of protein, DNA, RNA, and enzymes. Since elemental or pure nitrogen cannot be directly utilized by most living organisms. 2. Nitrification is the process by which nitrogen becomes available for plants. 3. Nitrification plays an important role in agricultural fields to increase the yield of essent…
Summary
- The Nitrogen cycle is also known as a perfect cycle in the biosphere. This is because it maintains the total amount of nitrogen present in the environment, soil and water. Nitrogen is part of amino acids and proteins, which are the building blocks of life. Atmospheric nitrogen needs to be converted into its usable forms through nitrification, ammonification and denitrification regularl…
Frequently Asked Questions (FAQs) on Nitrification
- Q.1. What conditions are needed for nitrification? Ans: The conditions needed for nitrification are: a. Moisture content b. pH between \(7-9\) c. Temperature between approximately \({\rm{10 – 3}}{{\rm{5}}^ \circ }{\rm{C}}\) d. Soil Retention Time(SRT) e. Aeration f. Soil matrix Q.2. Why does nitrification lower pH? Ans:Nitrification lowers the pH because most aquatic life cannot tolerate …