Complication rates were related to location of ablation and type of energy used. Individual complications were as follows: an average of 7.1% for access site complications defined as bleeding, hematoma, shock, infection, and vascular complications. Pericardial effusion occurred in 3.5%, pneumonia in 0.8%, stroke in 0.6%, and AV block III° in 0.3%.
What is the complication rate of PVC ablation in EPI location?
It was reported that the overall complication rate of PVCs ablation varied from 3.1 to 5.2% and complication rate of PVCs ablation in EPI location was higher than other locations [ 4, 5 ]. However, due to insufficient patient sample, predictors of PVC ablation were not evaluated in these studies.
What are the possible complications of cardiac ablation?
Complication rates were related to location of ablation and type of energy used. Individual complications were as follows: an average of 7.1% for access site complications defined as bleeding, hematoma, shock, infection, and vascular complications. Pericardial effusion occurred in 3.5%, pneumonia in 0.8%, stroke in 0.6%, and AV block III° in 0.3%.
When is ablation indicated for patients with PVCs?
Locations of left ventricle and epicardium were predictors of procedural complications for patients with PVCs. Therefore, ablation is not recommended in these patients. For other origins of PVCs, particularly RVOT origin, ablation is a safety and effective treatment.
What is the success rate of PVC ablation?
Outcomes. Ablation of outflow tract or fascicular PVCs is reportedly successful in 80% to 100% of cases. 13, 33 In two-thirds of the patients undergoing PVC ablation due to PVC-mediated cardiomyopathy, LV function improves to normal within 4 months, although in some cases it takes more than a year.

What is the success rate of ablation for PVCs?
Ablation of outflow tract or fascicular PVCs is reportedly successful in 80% to 100% of cases. 13,33 In two-thirds of the patients undergoing PVC ablation due to PVC-mediated cardiomyopathy, LV function improves to normal within 4 months, although in some cases it takes more than a year.
What can go wrong with catheter ablation?
Catheter ablation is a safe, effective treatment for AFib and certain other arrhythmias. Although rare, the risks of these procedures include: Bleeding, infection, and/or pain where the catheter was inserted. Blood clots (rare), which can travel to the lungs or brain and cause stroke.
How common are complications from cardiac ablation?
The overall complication rate of cardiac ablation has been shown to be 6.29%. Most common complications of procedure were cardiac (2.65%), vascular (1.33%), and neurological (1.05%) as shown in Table 1. According one study, cardiac tamponade was the most common complication with a rate of 1.31%.
Is ablation good for PVCs?
Ablation of PVCs in patients with normal LV function has become a safe and effective therapy for the treatment of patients with symptomatic PVCs,1 and as a result, PVC ablation holds a class 1 indication for treating frequent idiopathic symptomatic PVCs.
Is cardiac ablation worth the risk?
Ablation can relieve symptoms and improve the quality of life in people with atrial fibrillation. But it doesn't work for everyone. If atrial fibrillation happens again after the first ablation, you may need to have it done a second time. Repeated ablations have a higher chance of success.
What are the dangers of cardiac ablation?
Risks of Cardiac Ablation Damaged blood vessels if the catheter scrapes them. Arrhythmias caused by damage to your heart's electrical system. Blood clots in your legs or lungs. Heart damage, like punctures or damaged valves.
How long does it take for the heart to heal after an ablation?
The ablated (or destroyed) areas of tissue inside your heart may take up to eight weeks to heal. You may still have arrhythmias (irregular heartbeats) during the first few weeks after your ablation. During this time, you may need anti-arrhythmic medications or other treatment.
How many years does an ablation last?
Long-term success of AF ablation procedures, defined as freedom from arrhythmia recurrence for a minimum of 36 months off antiarrhythmic therapy, can be achieved in many patients.
Does heart ablation shorten life span?
Long-term survival is similar for patients with atrial fibrillation, whether they receive ablation or drug therapy. Control of the ventricular rate by ablation of the atrioventricular node and permanent pacing does not adversely affect long-term survival.
Do PVCs come back after ablation?
Recurrence of the predominant PVC shortly after ablation did not indicate a procedural failure and the necessity for a repeat procedure. The majority of these patients had a significant, clinically meaningful reduction in their PVC burden.
What happens after cardiac ablation for PVCs?
Some people feel a little sore after the procedure. The soreness shouldn't last more than a week. Most people can return to their daily activities within a few days after having cardiac ablation. Avoid heavy lifting for about a week.
How long does PVC ablation take?
The Procedure Catheter ablation can take between two and four hours to complete. The procedure is done in an electrophysiology lab where you will be monitored closely. Before the procedure begins, you will be given intravenous medications to help you relax and even fall asleep.
What is the complication rate of left atrial ablation?
The results came as a shock to many: the rate of overall complications from left atrial ablation (ie, pulmonary vein isolation/AF ablation) was 11.7% to 13.5% with a major complication rate of 3.8% to 7.2%.
What is the rate of complication with atrial flutter ablations?
Not surprisingly, overall rates of complication with atrial flutter ablations (which are usually right-sided) were lower than those seen with left atrial ablations, at 10.5%. However, it was surprising that major complications were more frequent with right atrial flutter ablations (7.4%), with an in-hospital death rate nearly 4 times as common vs left atrial ablations. There appeared to be an inflection point with respect to complication rates based on the experience of the performing center, with a 1.3 times higher rate in lower volume centers (≤100 left atrial ablations annually) and most of the differences being related to less serious complications, such as vascular access.
Is ablation safe for AF?
With the recent publication of the CABANA trial results, there has been a lot of enthusiasm for offering ablation as a first-line therapy for atrial fibrillation (AF). However, as our knowledge of (and expertise with) ablation evolve, we are appreciating that even procedures once perceived as relatively safe (based on analyses from retrospective studies and clinical trial data) carry significant complication rates when analyzed in a “real world” setting.
How does a catheter ablation work?
To perform an ablation, a catheter is fed through a vein to the heart, and the area of the heart that is causing the premature contraction is destroyed , or ablated , using radiofrequency . In recent years, advances in catheter ablation treatment have increased the safety and effectiveness of treating PVCs. Mapping technologies using new catheters that integrate with computer programs give surgeons a precise look at the areas of the heart causing the premature contraction and aid in finding and resolving the problem.
What are some ways to treat PVCs?
General treatments for PVCs. Many times, treatment options for PVCs include medication and lifestyle changes, as lifestyle factors can impact PVCs. For example, reducing stress and decreasing caffeine intake could lessen the occurrence of PVCs.
How to diagnose premature ventricular contractions?
If your doctor suspects you may have premature ventricular contractions, they will begin by ordering an Electrocardiogram (ECG) to rule out more serious diseases. Because PVCs do not occur all the time, an in-office ECG may not record the arrhythmia as it happens. For this reason, a Holter monitor that is worn for 24 to 48 hours is often used to diagnose PVCs. During this time, it continuously records the heartbeat, and the patient keeps a log of how they are feeling to match any recorded arrythmias with symptoms experienced by the patient. A doctor then reviews the data to diagnose premature ventricular contractions.
What is the purpose of ablation?
Using Ablation to Treat Premature Ventricular Contractions. Premature ventricular contractions (PVCs) are a common type of arrhythmia where the bottom chambers of the heart (the ventricles) cause extra beats. Typically, each beat of the heart begins in the sinus node in the upper chamber (the atria) of the heart.
Can PVCs be skipped?
For the patient, this arrhythmia will feel like a very hard heartbeat followed by a skipped or missed beat. PVCs can develop at any time and in all ages. PVCs can occur in otherwise healthy individuals with no other heart problems or in conjunction with other heart diseases.
Why is ablation needed for PVCs?
Suppression of PVCs with ablation is often required to potentially reverse the cardiomyopathy. PVCs are culpable in preventing recovery of LV dysfunction in patients with ventricular conduction abnormalities treated with cardiac resynchronization therapy (CRT). Reduction in delivery of true biventricular pacing with CRT to < 90% due to any reason, including a high burden of PVCs, is associated with a lack of improvement in LV systolic function.37
What is the best treatment for PVCs?
Medications can be used to suppress PVCs and are often employed as the first-line option. Drug selection is often based on the underlying cause and mechanism of PVCs, and the potential for adverse events. Outflow tract PVCs tend to respond to beta-blockers and calcium channel-blockers.33Idiopathic fascicular PVCs are particularly sensitive to verapamil. Rarely, sotalol or amiodarone may be required for idiopathic outflow tract or fascicular PVCs. Sodium channel-blockers (class I antiarrhythmics) flecainide and mexiletine can inhibit PVCs triggered by delayed after depolarizations. Reentrant PVCs are suppressed with class III antiarrhythmics including sotalol, dofetilide, and amiodarone, though class I agents also have a complementary role. Medical therapy is, however, limited by lack of efficacy for many patients. Additionally, beta-blockers, calcium channel-blockers, and sodium channel-blockers might cause unacceptable adverse effects such as fatigue or reduced ventricular inotropy and are difficult to take regularly on a long-term basis. Furthermore, class I (e.g., flecainide) and class III (e.g., sotalol and dofetilide) antiarrhythmic drugs have the risk of life-threatening proarrhythmia. In light of these shortcomings, patients may choose to pursue catheter ablation of PVCs implicated in causing symptoms, ventricular tachyarrhythmia, or cardiomyopathy.38,39
What is the first beat of a PVC?
The first beat is a sinus beat with proximal to distal activation of the fascicular system preceding the QRS complex. The second beat is the PVC with reversal of fascicular activation from the distal to the proximal bipole of the quadripolar-mapping catheter. The conduction time from the focus (sharp fascicular signal on ABL d) to the surface QRS is constant during sinus rhythm and PVC (24 ms). At more proximal sites (ABL, His), the fascicular signal to QRS time becomes shorter with PVC beat.
What tissue is used to trigger a PVC?
The His-Purkinje (fascicular) tissue can give rise to focal (likely triggered automaticity) PVCs or ventricular tachycardia or serve as circuits for reentry. The fascicular tissue is covered with insulation that prevents activation spread to the contiguous myocardium. Thus, PVCs originating within the fascicular system exit to the ventricular myocardium at multiple distant “breakout” sites, and multiple similarly early distant ventricular sites may be mapped. Pace match may not be optimal at any site due to multiple exits through the fascicular system, and even an excellent surface QRS match at best can identify only the breakout site. Paced morphology at the arrhythmogenic focus is different than the clinical PVC because local myocardium is captured before the fascicular tissue. Due to these limitations, classic mapping maneuvers like entrainment, pace mapping, and activation mapping are of limited utility, and sorting out ablation target sites can be challenging (Table 4).18,19,49In such cases, early high-frequency fascicular signals are of particular interest, and their activation needs to be annotated either in the same or a separate activation map. Relative timing of the early local fascicular signal-to-surface QRS complex during sinus rhythm and PVCs can identify the “focal” arrhythmogenic site. The most proximal site with a constant fascicular electrogram-to-surface QRS timing during PVCs and sinus beats is a good target. Further proximal sites will have a shorter electrogram-to-QRS time during PVCs when compared to sinus rhythm, because activation from a distal focus simultaneously activates the retrograde fascicular system and travels anterogradely to break out to the myocardium and generate the surface QRS complex (Figure 4).50Fragmented pre-Purkinje potentials with reversal of sequence during sinus beats are associated with small reentrant circuits involving the fascicular tissue and are potential targets of ablation.18,19It is worth pointing out that arrhythmogenesis of such fascicular PVCs may involve prolonged conduction zones close to the fascicular system interfacing with the adjacent myocardium, and the local fascicular signal may or may not participate in the PVC mechanism. In such cases, both the fascicular signals and any adjacent fragmented myocardial signals might be potential targets of ablation.19,51However, earliest pre-Purkinje potentials may be recorded quite proximally in the His-Purkinje system with a high risk of AV block with ablation at such sites, and it may be prudent to initially target more distal prepotentials.18,19Ablation targeting the fascicular signals in the posteroseptal region may lead to exit of the PVCs using other fascicles and change in QRS axis.52Successful ablation of fascicular PVCs may require empiric ablation of Purkinje (and pre-Purkinje) signals and adjacent ventricular myocardium in the region of interest (e.g., at the left midventricular posteroseptal region). If mapping and targeted ablation of left posterior fascicular PVCs is not possible or is unsuccessful, an empiric linear ablation transversely in the posteroseptal region, halfway between base and apex, has been described as an effective technique.53This usually transects the arrhythmogenic substrate and eliminates PVCs. Radiofrequency ablation of the fascicular tissue can elicit automaticity and induce refractory ventricular fibrillation.18,20
What is the QRS pattern of ventricular activation?
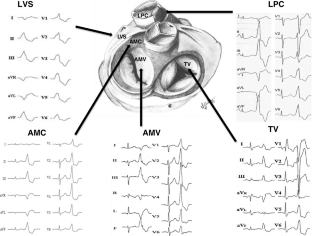
A point source origin of ventricular activation has a characteristic QRS pattern on ECG as the electrical wavefront traverses the contiguous myocardium and tracks around anatomic boundaries to activate both the ventricles in a predictable manner. Thus, the QRS morphology on ECG can predict the PVC's site of origin or exit to the larger myocardial mass (Table 1).13The site of origin or earliest activation is the target of ablation for focal PVCs; for reentrant PVCs, the critical arrhythmogenic tissue can often be targeted in proximity to the exit site. Outflow tract PVCs typically have an inferior frontal plane axis with tall positive R waves in inferior leads (II, III, aVF) and QS complexes in aVR and aVL.4Positive or negative QRS polarity in lead I can help localize PVCs to the right versus left parts of the outflow tracts. PVCs originating in the para-Hisian region close to the RV inflow are positive in leads I and aVL, whereas lead I is negative with leftward origin above the pulmonary valve or left coronary cusp. PVCs originating anteriorly from the free wall of the RVOT are wider compared to those from the septum and have left bundle branch block (BBB) morphology (QS in lead V1 with precordial R/S transition ≥ V3). Lead V1 starts to show R waves with PVCs originating further cephalad and leftwards close to the pulmonary valve, or posteriorly from the septum or adjacent locations in the LVOT including the right coronary cusp. PVCs from progressively posterior locations of the left coronary cusp, the noncoronary cusp, and aortomitral continuity (qR in V1) have progressively taller R waves in V1, and PVCs from the mitral annulus have a completely positive R wave in V1 (Figure 1). LVOT PVCs typically have an earlier precordial R/S transition (≤ V3) compared to RVOT PVCs.21PVCs originating from epicardial sites have a pseudo-delta wave, with slurring of the initial part of the QRS complex and delayed intrinsicoid deflection in the precordial leads, and can often be accessed through epicardial veins close to the great cardiac vein-anterior interventricular vein (GCV-AIV) junction.22,23
Can a PVC cause palpitations?
Though PVCs are fairly infrequent and asymptomatic in most cases, in some patients they may be more frequent and cause symptoms including palpitations, chest pain, and dyspnea. The spectrum of “benign” outflow tract PVCs ranges from single PVCs to repetitive nonsustained VT to paroxysmal sustained VT.29In rare cases, short-coupled RVOT PVCs can trigger polymorphic VT,27while even shorter-coupled PVCs often originating from the fascicular system or papillary muscles can trigger ventricular fibrillation.20,28PVCs in severe bileaflet mitral valve prolapse have been shown to be antecedents of malignant ventricular tachyarrhythmia.9PVCs are the frequent trigger for reentrant VT in patients with structural heart disease. PVC suppression with catheter ablation becomes necessary in such situations to prevent recurrent sudden cardiac arrest and implantable cardioverter defibrillator shocks.
Is PVC ablation a class IIA?
According to the American College of Cardiology/American Heart Association/European Society of Cardiology 2006 Guidelines, PVC ablation is reasonable (Class IIa) for symptomatic PVCs when drug therapy is ineffective, not tolerated, or not preferred by the patient. PVC ablation may also be considered (Class IIb) to treat or prevent PVC-mediated cardiomyopathy or for fascicular system PVCs that reproducibly induce ventricular fibrillation (Table 3).40
What is the procedure to remove PVCs?
Radiofrequency catheter ablation. For PVCs that don't respond to lifestyle changes or medications, your doctor might recommend ablation therapy. This procedure uses radiofrequency energy to destroy the area of heart tissue that is causing your irregular contractions.
How to control PVCs?
The following self-care strategies can help control PVCs and improve your heart health : Track your triggers. If you have frequent symptoms, you might want to take note of your symptoms and your activities. This can help identify substances or actions that may trigger premature ventricular contractions.
What causes premature ventricular contractions?
Caffeine, alcohol, tobacco and other recreational drugs are known triggers of premature ventricular contractions. Reducing or avoiding these substances can reduce your symptoms. Manage stress. Anxiety can trigger abnormal heartbeats.
How long do you need to monitor for PVCs?
In such cases, you may need to use a portable monitoring device for 24 hours or more to capture any abnormal rhythms. Common types of portable ECGs include:
Can you get PVCs with a normal heart?
Treatment. For most people, PVCs with an otherwise normal heart won't need treatment. However, if you have frequent PVCs, your doctor might recommend treatment. In some cases, if you have heart disease that could lead to more-serious rhythm problems, you might need the following: Lifestyle changes.
Why are catheter ablation rates less consistent?
In SHD, data on complication rates of catheter ablation for VT are less consistent because of the broad spectrum of underlying conditions and comorbidities, different strategies of mapping and ablation, and more frequent procedures in the setting of electrical storm. Generally, we can get an impression of complication rates from 2 sources. One is multicenter trials on catheter ablation for VT, and the other is experience from expert centers.
What is catheter ablation?
In structural heart disease, catheter ablation is predominantly used as an adjunctive treatment for recurrent VT in patients with implantable cardioverter-defibrilla tor and is often the treatment of choice for incessant VT or in electrical storms.
How is radiofrequency ablation applied?
In focal arrhythmias, radiofrequency current was applied at sites of earliest activation. In re-entrant arrhythmias, radiofrequency applications targeted primarily central or central-to-exit zones of slowly conducting channels. Deployment of the lesions was confirmed by subsequent noncapture at given sites. Additional lesions were applied in areas of late or fractionated potentials for arrhythmogenic substrate modification. The goal of ablation was to abolish all inducible VTs. Ablation was performed in power control mode with an irrigation flow of 30 mL/min. Power was set up to 20 to 40 W, depending on location and catheter contact, and was downregulated when catheter tip temperature rose >43°C or with a rapid drop of impedance (>10–15Ω) during ablation. Radiofrequency energy was applied in the majority of cases for a maximum of 60 seconds per target site. In patients where an epicardial approach was used, coronary angiography was performed to avoid application of radiofrequency energy in the vicinity of the epicardial coronary vessels. In the region of aortic cusps, power output was set in the majority of cases at 25 W. All of these cases were guided by intracardiac echocardiography; no coronary angiography was needed to identify ostia of the coronary vessels.
When is catheter ablation indicated?
In the vast majority, catheter ablation is indicated as adjunctive treatment when therapies from implantable cardioverter-defibrillator occur, frequently in the setting of electrical storm. In addition, the substrate for VT in patients with SHD is usually complex and often requires more extensive ablation.
When was the VT catheter ablation study performed?
The study included all consecutive VT catheter ablation procedures performed at our institution between August 2006 and December 2012. Occurrence of complications was evaluated <1 month after the procedure. The study was approved by the institutional review committee. Survival in all patients was obtained at 7 days from in-hospital notes and at 30 days from national citizen registry.
Is catheter ablation a treatment modality?
Catheter ablation has become an established treatment modality for a broad spectrum of ventricular tachycardias (VTs). We analyzed incidence and predictors of major complications of VT ablation procedures in a high-volume expert center.
Is there a risk of complications with catheter ablation?
It is generally accepted that the risk of complications is very low when catheter ablation is performed in idiopathic VTs. In a large series from an expert center, 5 the risk of major complications was ≈3%. Our data are fully in line with this observation. Importantly, there were no cardiac perforations and only 1 transient ischemic attack. Such figures are reassuring when patients with prognostically benign VTs are indicated for catheter ablation.
What is the treatment for PVCs?
Ablation is another treatment option for some patients with frequent or prolonged PVCs. In ablation therapy, radiofrequency waves are used to vaporize tiny amounts of tissue in the area of the heart where the extra beat originates. This is a minimally invasive procedure that is reserved for patients who cannot tolerate beta blockers, in whom medication is ineffective or who cannot comply with long-term drug therapy. Cleveland Clinic interventional cardiologists are experts in radiofrequency ablation , performing more than 1200 ablations annually to treat a variety of arrhythmias.
Why do PVCs go away?
When PVCs are due to some form or heart disease or structural abnormality, treating that problem often causes the PVCs to go away. A beta blocker medication may be prescribed for patients with PVCs who have heart failure or who have had a heart attack.
Why are PVCs so difficult to diagnose?
PVCs can be difficult to diagnose because they occur at unpredictable intervals. In most cases, PVCs are difficult for the physician to detect during a routine physical unless the patient has one during the exam or has other signs of structural heart problems. In individuals without any known heart disease, PVCs often are discovered incidentally during a routine electrocardiogram (EKG) In patients with known heart disease, PVCs may be detected during other diagnostic testing for that condition.
What is premature ventricular contraction?
What are premature ventricular contractions? A premature ventricular contraction (PVC) is a too-early heartbeat that originates in the ventricles and disrupts the heart’s normal rhythm. The pattern is a normal beat, an extra beat (the PVC), a slight pause, then a stronger-than-normal beat.
What is the number for the section of electrophysiology and pacing?
Section of Electrophysiology and Pacing: cardiology evaluation for medical management or electrophysiology procedures or devices - Call Cardiology Appointments at toll-free 800.223.2273, extension 4-6697 or request an appointment online.
How to control PVCs?
You can help control your PVCs by reducing or eliminating your caffeine, tobacco and alcohol intake and reducing stress and anxiety.
Do older people have PVCs?
PVCs occur more commonly in older people and in individuals with underlying heart disease , including a history of heart attack. People with a family history of cardiac arrhythmias (abnormal heart rhythm) also have a higher risk for PVCs.
Why does absence of PVC occur intermittently?
It is important to note that the absence of a PVC may occur intermittently because of ventricular refractoriness related to the separate underlying rhythm, and therefore multiples of the parasystolic PVC interval should be considered before excluding automaticity.
What are the causes of PVCs?
Premature ventricular complexes (PVCs) are extremely common, found in the majority of individuals undergoing long-term ambulatory monitoring. Increasing age, a taller height, a higher blood pressure, a history of heart disease, performance of less physical activity, and smoking each predict a greater PVC frequency. Although the fundamental causes of PVCs remain largely unknown, potential mechanisms for any given PVC include triggered activity, automaticity, and reentry. PVCs are commonly asymptomatic but can also result in palpitations, dyspnea, presyncope, and fatigue. The history, physical examination, and 12-lead ECG are each critical to the diagnosis and evaluation of a PVC. An echocardiogram is indicated in the presence of symptoms or particularly frequent PVCs, and cardiac magnetic resonance imaging is helpful when the evaluation suggests the presence of associated structural heart disease. Ambulatory monitoring is required to assess PVC frequency. The prognosis of those with PVCs is variable, with ongoing uncertainty regarding the most informative predictors of adverse outcomes. An increased PVC frequency may be a risk factor for heart failure and death, and the resolution of systolic dysfunction after successful catheter ablation of PVCs demonstrates that a causal relationship can be present. Patients with no or mild symptoms, a low PVC burden, and normal ventricular function may be best served with simple reassurance. Either medical treatment or catheter ablation are considered first-line therapies in most patients with PVCs associated with symptoms or a reduced left ventricular ejection fraction, and patient preference plays a role in determining which to try first. If medical treatment is selected, either β-blockers or nondihydropyridine calcium channel blockers are reasonable drugs in patients with normal ventricular systolic function. Other antiarrhythmic drugs should be considered if those initial drugs fail and ablation has been declined, has been unsuccessful, or has been deemed inappropriate. Catheter ablation is the most efficacious approach to eradicate PVCs but may confer increased upfront risks. Original research remains necessary to identify individuals at risk for PVC-induced cardiomyopathy and to identify preventative and therapeutic approaches targeting the root causes of PVCs to maximize effectiveness while minimizing risk.
How to diagnose PVC?
PVCs can be diagnosed only by ECG. The 12-lead ECG is useful to provide the initial evidence of PVC frequency and remains the best noninvasive tool to determine the PVC location. When a PVC is suspected from either the history or physical examination, it is useful to continue to run a rhythm strip over 30 to 50 seconds in the hopes of determining a better sense of PVC frequency and to catch the PVC during recording of all 12 contemporaneous leads to allow for the most accurate morphology assessment. Attention to the remainder of the ECG may also reveal clues to the underlying substrate: careful measurement of the QT interval is mandatory, precordial T-wave inversion beyond lead V 2 or right ventricular conduction delay may be indicative of arrhythmogenic right ventricular dysplasia, 30 pathological Q waves can reflect discrete areas of scar, an early precordial transition accompanied by a prominent S wave in V 6 may signal a basolateral scar observed in nonischemic cardiomyopathy, 31 and conduction disease may be a manifestation of cardiac sarcoidosis. 32 It is also important to emphasize that PVCs arising from common sites, such as the right ventricular outflow tract, may commonly trigger ventricular fibrillation in patients with the Brugada syndrome 33 and torsade de pointes in patients with the long-QT syndrome. 33, 34
Where is the most common PVC?
PVCs from the outflow tract appear to be the most common. 1, 16 Outflow tract PVCs characteristically exhibit negative QRS complexes in both aVL and aVR, consistent with a vector that is predominately arising from the top of the heart, and, by the same token, the inferior leads will all be positive. Right ventricular outflow tract PVCs will have a left bundle-branch morphology, meaning a predominately negative QRS in V 1. If the precordial transition (the precordial lead that first exhibits a QRS that is more positive than it is negative) occurs at V 4 or later and the other limb-lead criteria just described are present, the PVC is almost certainly arising from the right ventricular outflow tract. 16, 36 If an otherwise similar PVC has a precordial transition that occurs in V 1 or V 2, it is almost certainly left-sided, most likely arising from the right or left coronary cusp (or just in-between the 2). If an otherwise similar PVC precordial transition occurs at V 3, it may be either right or left sided. 16, 36 The distinction is relevant to both the effectiveness and the risks of potential catheter ablation, which, as will be described in more detail later in this article, are both more favorable for right-sided PVCs. Understanding these morphological characteristics is also relevant because right ventricular PVCs that are not arising from the right ventricular outflow tract may be a sign of underlying pathology, such as arrhythmogenic right ventricular dysplasia, sarcoidosis, or other infiltrative diseases. For example, although idiopathic PVCs may arise from various locations within the right ventricle in the absence of structural heart disease, a frequent left bundle-branch, late-precordial transition (more positive than negative starting in V 4) PVC that is not negative in both aVR and aVL and that is negative in the inferior leads should prompt further evaluation with cardiac magnetic resonance imaging (MRI). Commonly observed PVC morphologies from the left ventricle include the papillary muscles, 37–39 which may often exhibit variable morphologies within the same patient, the left anterior or left posterior fascicles (which may occur because of either reentry or automaticity), 40 and along the mitral annulus. 41–43 PVCs also arise relatively frequently in proximity to venous structures, such as the great cardiac vein and anterior intraventricular vein 44–46 and from the crux of the heart, where all 4 chambers meet. 47
What is the first indication of PVC frequency?
Similarly, that physical examination may be the first indication of PVC frequency, which ultimately may prove to be clinically relevant.
Is premature depolarization a mechanical contraction?
Of note, the Heart Rhythm Society consensus recommends the term “premature ventricular complex” and not “ventricular premature depolarization” or “premature ventricular contraction” to standardize the literature and acknowledge that electric activity may not lead to mechanical contraction. 1.
Is a PVC asymptomatic?
PVCs are commonly asymptomatic but can also result in palpitations, dyspnea, presyncope, and fatigue. The history, physical examination, and 12-lead ECG are each critical to the diagnosis and evaluation of a PVC. An echocardiogram is indicated in the presence of symptoms or particularly frequent PVCs, and cardiac magnetic resonance imaging is ...
