What is the quantity of alum used to treat water?
- Alum is a safe and effective lake management tool.
- Alum applications should be designed and controlled to avoid concerns with toxicity to aquatic life.
- Watershed management is an essential element of protecting and managing lakes. ...
Is alum in water a true solution?
a true solution of common salt, sugar and alum in water. a suspension of soil, chalk powder and fine sand in water. True solution: A solution that has solute particles of size smaller than 1 nm (10 -9 metres) in diameter, and cannot be seen with naked eyes.
What is alum good for?
Your data can be used to monitor for and prevent fraudulent activity, and ensure systems and processes work properly and securely. Your device can receive and send information that allows you to see and interact with ads and content. Different devices can be determined as belonging to you or your household in support of one or more of purposes.
Can potassium alum be used to purify water?
The MarketWatch News Department was not involved in the creation of this content. Jul 12, 2021 (Heraldkeepers) -- Potassium alum is a chemical compound that can be used to purify water, tanned leather, as a hair powder or in place of aluminum sulfate as a vanamin blue dye inhibitor.
Is alum good for water purification?
As a water purifier: Alum is one of the most ancient ways to ensure that drinking water is clean. A pinch of alum added to water removes the solid impurities. Once the sediment is thrown away, the water is boiled to kill bacteria.
Why is alum Aluminium sulfate added during water treatment?
When added to water, aluminum sulfate causes microscopic impurities to clump together into larger and larger particles. These clumps then settle to the bottom of the container and can be filtered out. This makes the water safer to drink.
Does alum reduce pH?
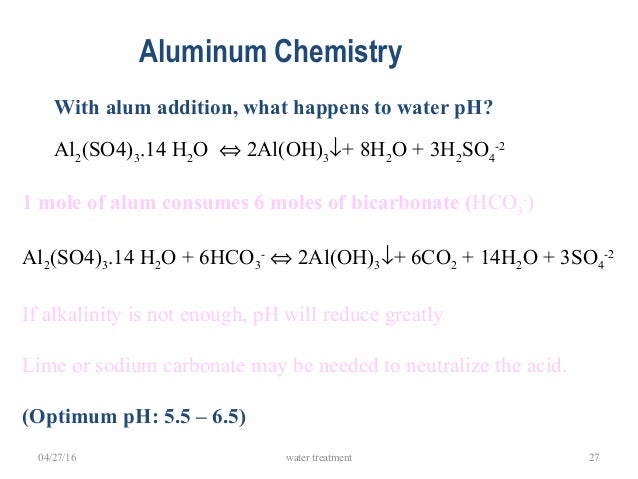
Alum (aluminum sulfate; Al2(SO4)3. 14H2O) is acidic in water and can reduce total alkalinity and pH by neutralizing carbonate and bicarbonate compounds with a greater decline in pH when applied to water with low initial total alkalinity (Boyd 1979a; 1990; Wilkinson 2002).
What is the purpose of alum?
Alum (aluminum sulfate) is a nontoxic liquid that is commonly used in water treatment plants to clarify drinking water. It's use in lakes began in the early 1970's and is used to reduce the amount of phosphorus in the water.
What is a good substitute for alum?
Proprietary products, instead of commodities, may offer a better solution. For example, a few products from ATS Innova’s ATS 800 line are excellent substitutes for alum and ferric chloride: ATS 835 is a great replacement for alum because it does an amazing job, but with a much, much smaller dose. For example, a water treater who feeds 25 parts ...
What is a coagulant in water treatment?
As every water treater knows, coagulants are used in the first step of water treatment; they destabilize the water so micro flocks can begin to form. This is followed up by a polymer, which turns it into a larger flock by creating a bigger charge. Alum and ferric chloride are two widely-used “commodity chemical” coagulants ...
What is the best replacement for ferric chloride?
Both large and small plants will see cost savings; the more ATS 806 is used to replace ferric chloride, the larger the savings. It should be noted that both ATS 835 and 806 are potentially excellent replacements for alum or ferric chloride—it’s all dependent on the chemistry.
Why is sludge not easy to de-water?
It produces a lot of sludge. Plus, the sludge it creates is not very easy to de-water because it becomes very gelatinous. For example, let’s say a small plant runs at a million gallons a day, feeding 25 parts of alum on a wet basis.
Is ATS 835 soluble in water?
And because ATS 835 is completely soluble, it will hardly increase solids due to the coagulant. Alternatively, if a water treatment plant is using ferric chloride, a recommended replacement product is ATS 806.
Is ferric chloride a competitor for alum?
Some water treaters simply aren’t aware of alternative products. The companies that supply alum and ferric chloride aren’t advertising competing products, and the companies that do sell alternative products struggle to get the word out.
Can alum be used to treat dirty water?
To treat excessively dirty water, simply increasing the alum isn’t going to do anything. For example, to treat muddy water like that from the Mississippi, even 250 parts of alum isn’t going to cut it. Alum just doesn’t work in very dirty water. It produces a lot of sludge.
Why is alum used in watershed management?
Thus, the use of alum may be the only practical way to accomplish meaningful and timely water quality improvements. Using alum as an element of a comprehensive watershed and lake management program will often be needed to achieve meaningful results in a timely and cost-effective manner.
What happens when aluminum sulfate is added to water?
As aluminum sulfate is added to water, it forms aluminum ions, which are hydrated (combined with water): In a series of chemical hydrolysis steps, hydrogen ions are liberated, which may lower the water pH, and ultimately forms aluminum hydroxide (Al (OH) 3 ), which is a solid precipitate:
What is the name of the substance that is added to lake water to remove phosphates?
Aluminum sulfate, called alum, when added to lake water removes phosphates through precipitation, forming a heavier than water particulate known as a floc. This floc then settles to the lake bottom to create a barrier that retards sediment phosphorus release. There are two policy-related issues with the use of alum:
What is the effect of aluminum hydroxide blanket?
The aluminum hydroxide blanket, when applied appropriately, separates the sediment from the water column, which reduces internally supplied phosphorus. Free aluminum may persist at pH less than 6 or other hydroxides may form at pH greater than 9; although toxicity may occur at pH > 8 in some conditions.
Is alum a phosphorus?
For purposes here, alum is not considered an algaecide for the simple reason that any algae control effects following an alum application are the result of phosphorus reduction rather than any direct toxic effects on algae control.
Is alum safe to use in lakes?
Alum is a safe and effective method to mitigate excess phosphorus in lakes and reservoirs. Note, there are many other methods and approaches to consider in managing lakes (see Wagner 2001). The concerns with using alum cited here can be managed or balanced.
Does alum water treatment exceed drinking water standards?
Again, the raw water supply does not exceed drinking water standards shortly after an alum application.
What is alum water treatment?
Alum water treatment is generally carried to treat the polluted water. These compounds act as a coagulant. It is used in the coagulation-flocculation process of polluted water. It is a chemical water treatment technique typically applied prior to sedimentation and filtration to enhance the ability of a treatment process to remove particles.
What is the chemical formula for alum?
Alum is a double salt present in the hydrated form. The general chemical formula for alum is XAl (SO4) 2·12H2O.
Is alum soluble in water?
Yes, alum is soluble in water. Alum is an ionic compound consist of water of hydration. These large number of water molecules contribute to the solubility of alum in water. It gets completely dissociated when dissolving in water.
How to apply alum solution to a tank?
The most simple and common way to apply this solution is with a tank and a gas-powered trash pump. Use the pump to fill the tank with water, apply the alum, mix thoroughly, then use the pump to empty the tank into the waterbody. You will want to apply the alum solution proportionately to depth, applying more in the deeper areas.
What does alum do to a pond?
Due to the chemical makeup of alum, it will want to bind with a wide range of particles from clay sediment to nutrients such as nitrogen and phosphorus. When added to the water, the alum has a strong positive charge, and most raw water particles are negatively charged, so they bind very tight to each other. Fortunately, this chemical property solves two of the biggest problems pond owners have; eutrophication and clarity issues. In other words, waterbodies will accumulate and store whatever is washing into it, whether it be nutrients from fertilizer or clay particulates from runoff. Overtime your nutrient levels will become higher due to all the input with nothing balancing it out. Aluminum sulfate can essentially act as a reset button for your nutrient content, as well as any other sediment that might be floating in the water column. Conveniently, phosphorus and nitrogen are the two nutrients that algae and rooted plants rely on the most for growth. When the alum binds with these nutrients, it renders them useless, and if done correctly can prevent plant growth before it happens.
What happens when you mix alum with water?
When mixed with water, one of the byproducts of the alum will be sulfuric acid. Alkalinity is a measurement of how well your water can buffer changes in ph. These tests ensure that when the application occurs, your waterbody’s pH will not plummet when the alum is applied, potentially stressing the fish in the pond.
How to get the most out of alum?
To get the most out of your alum, you will want to premix your supply in a tank of water on a calm day. The premixed solution should be thoroughly agitated to ensure that the alum is free floating in the water. The most simple and common way to apply this solution is with a tank and a gas-powered trash pump.
What are the precautions when applying aluminum sulfate?
When applying alum, there are some parameters that need to be watched so we can be confident that the application will go smoothly. The first and most important tests you want to perform is the pH and alkalinity on the waterbody you are going to be applying the alum to.
How long does phosphorus stay in the water?
Ideally, you want the product to remain in the water column for several hours tying up excess phosphorus and sediment. Once on the bottom, it will tie up nutrients and sediment that are already resting but will not efficiently accumulate particles that are suspended.
What is aluminum sulfate used for?
Aluminum Sulfate, or alum, is a chemical that is very useful in the context of lake management. It is found in nature but can also be made in a lab very easily. It plays a role in several industries such as paper manufacturing and textile production, although one of the most common uses is in the water treatment industry as a coagulant.
Introduction
For a chemist like me, alums are an interesting group of chemical compounds, a large group of aluminium sulfates. Their name comes from the Latin word alumen meaning bitter salt, referring to one of them, potassium aluminium sulfate. Alums dissolve in water and they are quite widely found in nature.
Why Use Alum to Clarify Muddy Water?
Alums are useful as well as beautiful, and humans have been using them for millennia. For example, the Egyptians used an alum to help the yellow dye bind to the cotton cloth used to wrap their mummies. Gardeners add alum to the soil to help their Hydrangeas turn blue.
How to Treat Muddy Water With Alum
Collect 6 L of water in a bucket to treat at campsites. Mostly I use my own homemade buckets (e.g. Figs 2C, 3B & 3C), that are stabilized with side batons that prevent them from falling over. Commercial foldable buckets can also be used if they are stable, but often that requires securing them by the handle from above.
