Flocculants are used in many different types of processes, such as cheese-making and brewing. When it comes to water treatment processes, they are used to remove microscopic particles that can affect everything from taste to appearance, smell and texture. Flocculation
Flocculation
Flocculation, in the field of chemistry, is a process wherein colloids come out of suspension in the form of floc or flake; either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended in a liquid and not actually dissolved in a solution.
What do flocculants do in water treatment?
· Flocculation is a water treatment process where solids form larger clusters, or flocs, to be removed from water. This process can happen spontaneously, or with the help of chemical agents. It is a common method of stormwater treatment, wastewater treatment, and in the purification of drinking water. One of the requirements for treated water leaving wastewater …
What happens during the process of flocculation?
Flocculation or floculation is a significant process widely utilized in water treatment operations, including purification of drinking water, sewage water treatment, storm water treatment and treatment of various other industrial wastewater streams. Though often used interchangeably with coagulation, it is in fact a distinct process that similar to coagulation aids in sediment and …
What is added to water to cause flocculation?
Flocculants are used in many different types of processes, such as cheese-making and brewing. When it comes to water treatment processes, they are used to remove microscopic particles that can affect everything from taste to appearance, smell and texture. Flocculation is a mixing process with results in accelerated particle collision.
What is difference between flocculation and precipitation?
· In water treatment terms, flocculation is the process of encouraging the formation of clumps of solids — or flocs. This is necessary in the removal of solids that are suspended in the water, leading to an easier process of filtration. Flocculants, or flocculation agents, are deployed in the water to create these flocs and to accelerate the process where necessary.

What is flocculation in water treatment?
Flocculation or floculation is a significant process widely utilized in water treatment operations, including purification of drinking water, sewage water treatment, storm water treatment and treatment of various other industrial wastewater streams. Though often used interchangeably with coagulation, it is in fact a distinct process that similar to coagulation aids in sediment and contaminant transport. Usually, flocculation follows the coagulation process and helps in getting rid of of colloidal particles or flocs through rapid settlement in the solution. In certain cases, flocks also rise to the surface of the treated liquid, which can then be filtered out from the solution through the process of filtration.
What is flocculation used for?
Predominant uses of flocculation is in water and wastewater treatment, such as drinking water treatment, sewage water treatment, storm water treatment and treatment of various other industrial wastewater streams. Flocculation is also used in brewing, especially to measure the progress of brewing yeast at the end of fermentation.
What is flocculant in a fluid?
In the flocculation process, flocculants, or flocculating agents are generally added to the liquid so that flocculation is promoted and smaller particles (inorganic and organic), water-stable soil aggregates, or flocs/flakes agglomerate to form larger particles or lumps in the flowing medium.
What are the factors that determine the formation of flocs?
Formation of flocs or flakes during flocculation is a complex process that depends on several factors. A few important factors are as follows: Physical ( e.g., Turbulence) Chemical ( e.g., Ionic concentration) Biological (Bacterial populations and extracellular polymeric material). Uses of Flocculation. Predominant uses of flocculation is in water ...
Why is coagulation important?
Coagulation is also important in several wastewater treatment operations. A common example is chemical phosphorus removal and another, in overloaded wastewatertreatment plants, is the practice of chemically enhancing primary treatment to reduce suspended solids and organic loads from primary clarifiers.
What are some examples of coagulation operations?
Coagulation operations can be useful in some cases for the removal of inorganics. Examples of successful applications are copper and mercury reductions from wastewaterplant effluents. Two applications discussed in more detail below are arsenic and fluoride removals in potable water treatment:
How does temperature affect coagulation?
Temperature significantly affects coagulation operations, particularly for low turbidity waters, by shifting the optimum pH. This can be mitigated by operating at an optimum pOH as given by:
How much giardia is removed from water?
The U.S. EPA surface water treatment rule requires 99.9-percent (3-log) Giardia removal or inactivation, and at least 99-percent (2-log) removal of Cryptosporidium. Adequately designed and operated water treatment plants, with coagulation, flocculation, sedimentation and filtration are assigned a 2.5-log removal credit for Giardia, leaving only 0.5-log inactivation to be achieved by disinfection.
What is the best coagulant for organics removal?
Organics removal and enhanced coagulation are effective with traditional coagulants like aluminum sulfate, ferric chloride and ferric sulfate, as well as formulations like polyaluminum chloride (PACl) and acid alum. Acid alum formulations are aluminum sulfate with 1 to 15-percent free sulfuric acid.
Which is easier to remove: humic acid or fulvic acid?
In general, lower molecular weight species such as the fulvic acids are more difficult to remove by coagulation. Higher molecular weight humic acids tend to be easier to remove.
Does coagulation remove organic material?
The degree to which coagulation can remove organic material depends on the type of material present , as shown in Figure 2. The specific ultraviolet absorption (SUVA) is related to the concentration and type of dissolved organic carbon (DOC) present, as follows:
What are the factors to consider when choosing a water treatment system?
When considering different coagulant and flocculant options available for a given municipal or commercial water treatment system, there are many factors to consider in order to avoid wasting money on ineffective chemicals, surcharges and more . Raw water quality (for instance, alkalinity, water hardness, etc.), the available processing equipment and the treatment objectives (potable use or industrial use) must all be appraised in a unique case-by-case basis in order to choose the right combination of coagulants and flocculants. With proper care and consideration, a water treatment company can ensure that they’ve selected the most appropriate treatments available to service the needs of their local community, clients and customers.
Why are chemicals added to water?
Chemicals are then added to pre-treat the water, regulating pH levels and disinfecting the water in order to control of taste and/or smell as needed. Coagulants are then added in order to stimulate the formation of flocs.
Inorganic flocculants
Inorganic compounds are comprised of molecules that do not contain carbon. These compounds make up some of the most widely used flocculation agents on the market.
Organic flocculants
Organic flocculants include carbon-based molecules and may be biological in nature. The following agents may represent a more efficient alternative to inorganic flocculants in some cases.
What are the factors that affect the flocculation process?
Important factors include the pH of the water, its temperature, the time it spends in the flocculation basin and the type of coagulant chemicals employed .
What pH is needed for flocculation?
If the coagulant chemical is alum, for example, pH in the 4.4 to 6 range is optimal for flocculation. Higher temperature accelerates the process, which means that flocculation may be slower in the winter, so operators may need to allow more time when the weather is cold.
What is the process of removing suspended solids from water?
One of the first and most important steps in treatment is the removal of suspended solids, fine particles mixed into the water. Flocculation is a chemical and mechanical process that enables treatment plants to quickly remove solids from the water.
How long does it take for wastewater to mix?
Generally this initial flash mixing phase takes only a minute. Next the water is mixed gently in a flocculation basin while the solids settle to the bottom.
What are the two types of chemicals that are used in wastewater treatment?
The first is primary coagulants, chemicals with a positive charge that adhere to the negatively charged particles so they are now neutral and can come together. Alum and ferric sulfate are common examples. Coagulant aids are the second kind of chemical; these are agents that make the settling solids denser and thereby speed up the process, although they are not strictly necessary. Negatively charged anionic polymers are a common example.
Why is wastewater treated and disinfected?
Sewage is rich in all kinds of filth -- everything from household chemicals to human feces and dangerous bacteria. That's why it must be treated and disinfected before it can be discharged to the environment.
Does floc dissolve in water?
This floc contains not only suspended solids but also some bacteria and a variety of chemicals that dissolve poorly in water. Once a sufficient amount of time has elapsed, the water passes on to the next stage in treatment.
What are the steps of surface water treatment?
In this lesson students learn about the first three steps of a conventional surface water treatment plant: coagulation, flocculation and sedimentation. They learn the basic chemistry behind destabilizing natural water particles and the physics behind encouraging the collision of those particles to form flocs that will settle out of solution. Students acquire knowledge about the specifics of the three processes, while enforcing an understanding of how each process works within the overall design of water treatment.
What is the last process to the first barrier against water contamination?
The last process to the first barrier against water contamination is sedimentation . During sedimentation, the flow of the water is slowed to resemble a calm environment. As the water is calmed, the large flocs that have been formed settle to the bottom of the sedimentation basin, sometimes called a clarifier. As the flocs are settling to the bottom, the relatively particle free water passes over a system of weirs and moves to the filtration process.
Why are particles in water considered stable?
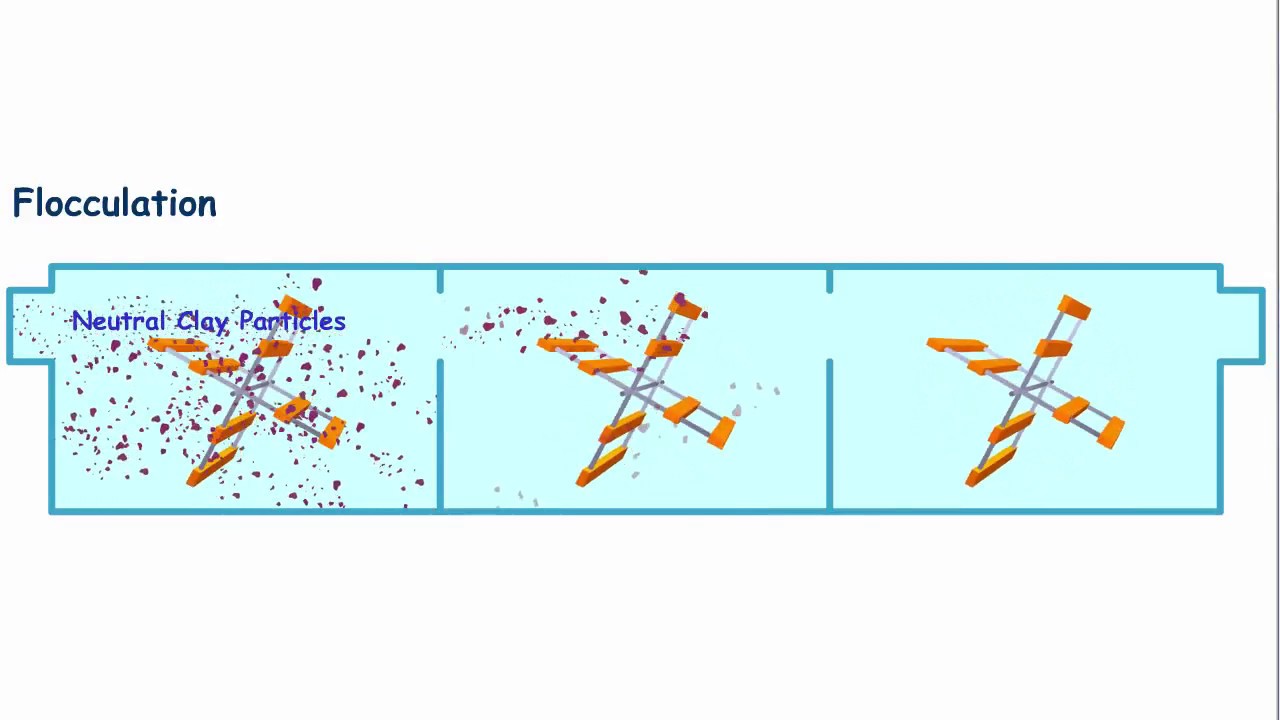
The cause of this stability is static electricity. Particles in natural waters are coated with macromolecules called ‘natural organic matter,’ which are produced by the decomposition of organic matter such as leaves, living organisms, aquatic plants, etc. Natural organic matter has functional groups that at neutral pH’s are negatively charged, giving the overall charge of the particles. Because all of the particles are negatively charge, they are repelled by one another so that the particles cannot collide and stick together to form larger and larger particles. Therefore, the goal of the first process in drinking water treatment, coagulation, is to destabilize the particles and allow them the potential to collide and stick together.
What are the three processes of treatment?
The first three processes of treatment make up the first barrier against contaminants. The main goal of coagulation, flocculation and sedimentation is to remove the particles, which could be harmful to human health, from the water. If done correctly, a majority of the incoming particles formed flocs and settled out in the sedimentation basin, yet there inevitably will be smaller particles or flocs that remain suspended in the water as it leaves. Although the probability of contaminates in the water leaving the sedimentation basin has been greatly reduced, to remove the remaining particles or flocs the water must be first passed through a media filter, which is the second barrier between potential pathogens and the consumer.

Coagulation and Flocculation in Water and Wastewater Treatment
The Coagulants
- The commonly used metal coagulants fall into two general categories: those based on aluminum and those based on iron. The aluminum coagulants include aluminum sulfate, aluminum chloride and sodium aluminate. The iron coagulants include ferric sulfate, ferrous sulfate, ferric chloride and ferric chloride sulfate. Other chemicals used as coagulants include hydrated lime and magn…
Removal of Natural Organic Matter
- Natural organic material (NOM) is usually associated with humic substances arising from the aqueous extraction of living woody substances, the solution of degradation products in decaying wood and the solution of soil organic matter. These substances are objectionable for a number of reasons: they tend to impart color to waters; they act as a vehicle for transporting toxic substan…
Pathogen Removal
- The U.S. EPA surface water treatment rule requires 99.9-percent (3-log) Giardia removal or inactivation, and at least 99-percent (2-log) removal of Cryptosporidium. Adequately designed and operated water treatment plants, with coagulation, flocculation, sedimentation and filtration are assigned a 2.5-log removal credit for Giardia, leaving only 0.5-log inactivation to be achieved by …
Removal of Inorganics
- Coagulation operations can be useful in some cases for the removal of inorganics. Examples of successful applications are copper and mercury reductions from wastewaterplant effluents. Two applications discussed in more detail below are arsenic and fluoride removals in potable water treatment:
Wastewater Treatment
- Physical-chemical treatment of wastewater was widely practiced until the late 19th century, until the advent of the trickling filter for biological treatment. The early 1970s saw a partial revival of interest that has continued to the present day, particularly for treatment plants that are overloaded during peak flow events. The addition of coagulant chemicals to primary clarifiers, or to other de…
Factors Affecting Coagulation Operations
- Temperature
Temperature significantly affects coagulation operations, particularly for low turbidity waters, by shifting the optimum pH. This can be mitigated by operating at an optimum pOH as given by: pH + pOH = pKW; where pKW = 0.01706xT + 4470.99/T – 6.0875 and T = temperature in °K = 273.15 … - Sequence of chemical addition
Traditionally, the sequence of chemical addition for coagulation operations is to first add chemicals for pH correction, then add the metal coagulant, then add the flocculant aid. Not all these chemicals are necessarily added, but the sequence logic is often as described. However, t…
Rapid Mixing
- The rapid mixing stage is possibly the most important component of coagulation-flocculation processes, since it is here that destabilization reactions occur and where primary floc particles are formed, whose characteristics markedly influence subsequent flocculation kinetics. In general it is likely that the metal coagulant hydrolysis products that are formed within the tim…
Flocculation
- Orthokinetic flocculation arises from induced velocity gradients in the liquid. It is here that primary particles are induced to approach close enough together, make contact and progressively form larger agglomerates, or flocs. The principal parameter governing the rate of orthokinetic flocculation is the velocity gradient applied. The degree or extent of flocculation is g…
Testing and Control
- The efficiency of the coagulation-flocculation process is dependent on many variables. For a particular water these may include: 1. Type of coagulant used 2. Coagulant dosage 3. Final pH 4. Coagulant feed concentration 5. Type and dosage of chemical additives other than primary coagulant (e.g. polymers) 6. Sequence of chemical addition and time lag between dosing points …