What is coagulation normal values?
Oct 15, 2021 · Coagulation treatment is usually carried out before sedimentation and filtration. During the process, a coagulant is added to water, and its positive charge neutralizes the negative charge of suspended contaminants. Neutralization causes suspended particles to bind together (hence the term). In clumps known as “flocs”, these particles sink ...
What are flocculants and coagulants for wastewater treatment?
What does a coagulation test determine?
What is the purpose of a coagulation test?

What is the purpose of coagulation in water treatment?
Coagulation is the chemical water treatment process used to remove solids from water, by manipulating electrostatic charges of particles suspended in water. This process introduces small, highly charged molecules into water to destabilize the charges on particles, colloids, or oily materials in suspension.6 days ago
What are the advantages of using a coagulant in water treatment?
The main advantage of using an organic coagulant is reducing sludge volume and bulk and thus reducing the amount of material to manage (i.e. de-water) or dispose of. These coagulants are very effective; however, as Kim points out, they are pretty pricey.
Why is coagulation and flocculation important in water treatment?
Coagulation and flocculation are essential components of both drinking water and wastewater treatment. They provide a reliable process for treating water turbidity (the cloudiness or haziness of a fluid typically invisible to the naked eye), which is a key test of water quality.
Why is chlorine such an important part of the treatment?
Chlorine's use has seen it help to virtually eliminate waterborne diseases, such as cholera, typhoid and dysentery in developed countries. It also eliminates slime bacteria, molds and algae that commonly grow in water supply areas, on the walls of water mains and in storage tanks.Jun 28, 2010
What are advantages of using sodium aluminate as coagulant in the water treatment process?
Key benefitsGives high purity and quality of water.Excellent coagulation, flotation and sedimentation.Increases alkalinity – no need for lime and hydroxides.Excellent removal of phosphor.Minimal chemical sludge.Low transportation cost.
Why coagulation is needed?
Coagulation is a process used to neutralise charges and form a gelatinous mass to trap (or bridge) particles thus forming a mass large enough to settle or be trapped in the filter.May 24, 2019
What is the purpose of coagulation in drinking water treatment quizlet?
The purpose of coagulation and flocculation is to remove particulate impurities and color from the water being treated.
Why is pH important for coagulation flocculation?
Since pH values affect the surface charges and forms of the coagulants and impurities to be removed, controlling the level of pH would significantly improve the coagulation process. Therefore, not only coagulant dosage, but also pH value should be optimized to maximize the removal of impurities present in raw water.Apr 5, 2019
What is the best coagulant for organics removal?
Organics removal and enhanced coagulation are effective with traditional coagulants like aluminum sulfate, ferric chloride and ferric sulfate, as well as formulations like polyaluminum chloride (PACl) and acid alum. Acid alum formulations are aluminum sulfate with 1 to 15-percent free sulfuric acid.
What are the variables in coagulation?
The efficiency of the coagulation-flocculation process is dependent on many variables. For a particular water these may include: 1 Type of coagulant used 2 Coagulant dosage 3 Final pH 4 Coagulant feed concentration 5 Type and dosage of chemical additives other than primary coagulant (e.g. polymers) 6 Sequence of chemical addition and time lag between dosing points 7 Intensity and duration of mixing at rapid mix stage 8 Type of rapid mix device 9 Velocity gradients applied during flocculation stage 10 Flocculator retention time 11 Type of stirring device used 12 Flocculator geometry.
How does orthokinetic flocculation work?
Orthokinetic flocculation arises from induced velocity gradients in the liquid. It is here that primary particles are induced to approach close enough together, make contact and progressively form larger agglomerates, or flocs. The principal parameter governing the rate of orthokinetic flocculation is the velocity gradient applied. The degree or extent of flocculation is governed by both applied velocity gradients and time of flocculation. These two parameters influence the rate and extent of particle aggregation and the rate and extent of breakup of these aggregates.
What happens when you add coagulants to water?
When metal coagulants are added to water the metal ions (Al and Fe) hydrolyze rapidly but in a somewhat uncontrolled manner, forming a series of metal hydrolysis species. The efficiency of rapid mixing, the pH, and the coagulant dosage determine which hydrolysis species is effective for treatment.
What is the rapid mixing stage?
The rapid mixing stage is possibly the most important component of coagulation-flocculation processes, since it is here that destabilization reactions occur and where primary floc particles are formed, whose characteristics markedly influence subsequent flocculation kinetics. In general it is likely that the metal coagulant hydrolysis products that are formed within the time range 0.01 to 1.0 seconds are the most important for effective destabilization. In many instances, traditional 30 to 60 second retention times during rapid mixing are unnecessary and flocculation efficiency may not improve beyond rapid mix times of approximately 5 seconds or less. Indeed, beyond a certain optimum rapid mix time, a detrimental effect on flocculation efficiency may result.
What are some examples of coagulation operations?
Coagulation operations can be useful in some cases for the removal of inorganics. Examples of successful applications are copper and mercury reductions from wastewaterplant effluents. Two applications discussed in more detail below are arsenic and fluoride removals in potable water treatment:
What is the sequence of chemical addition?
Traditionally, the sequence of chemical addition for coagulation operations is to first add chemicals for pH correction, then add the metal coagulant, then add the flocculant aid. Not all these chemicals are necessarily added, but the sequence logic is often as described. However, there are instances when other sequences are more effective, including inverting the sequence of metal coagulant and polymer addition, and the sequence of metal coagulant addition and pH adjustment. The best sequence for a particular application can be determined by jar test experiments.
Coagulation and Flocculation in Water and Wastewater Treatment
- Coagulation and flocculation are an essential part of drinking water treatment as well as wastewater treatment. This article provides an overview of the processes and looks at the latest thinking. Material for this article was largely taken from reference1. Coagulation and flocculation are essential processes in various disciplines. In potable water treatment, clarif…
The Coagulants
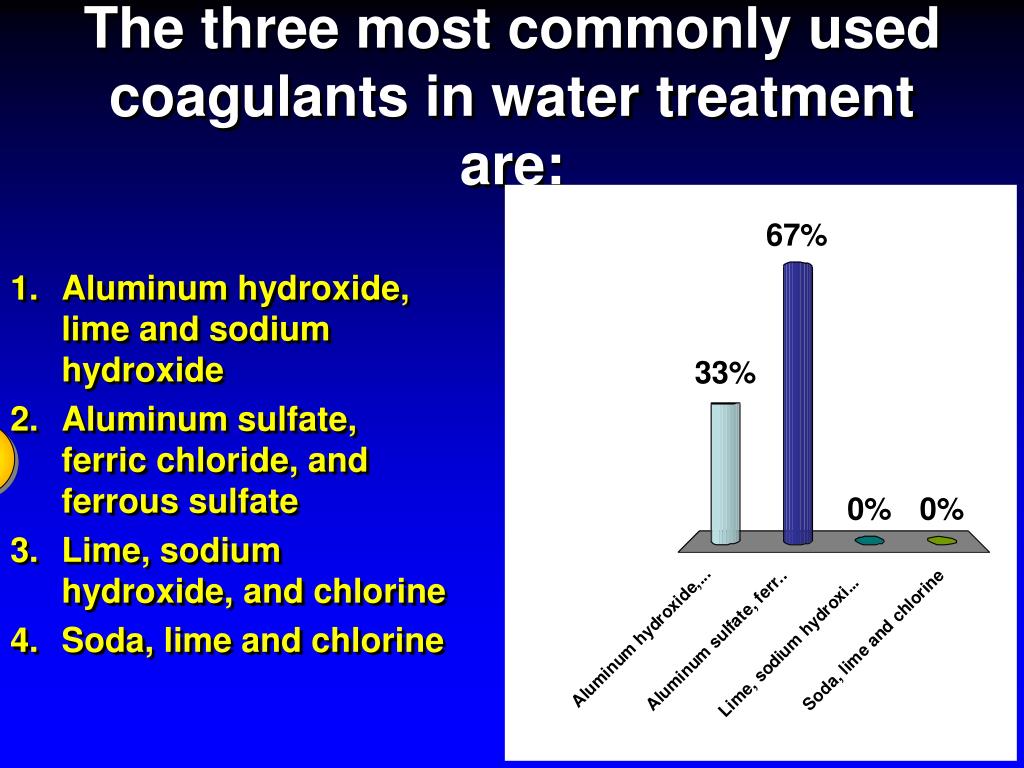
- The commonly used metal coagulants fall into two general categories: those based on aluminum and those based on iron. The aluminum coagulants include aluminum sulfate, aluminum chloride and sodium aluminate. The iron coagulants include ferric sulfate, ferrous sulfate, ferric chloride and ferric chloride sulfate. Other chemicals used as coagulants include hydrated lime and magn…
Removal of Natural Organic Matter
- Natural organic material (NOM) is usually associated with humic substances arising from the aqueous extraction of living woody substances, the solution of degradation products in decaying wood and the solution of soil organic matter. These substances are objectionable for a number of reasons: they tend to impart color to waters; they act as a vehicle for transporting toxic substan…
Pathogen Removal
- The U.S. EPA surface water treatment rule requires 99.9-percent (3-log) Giardia removal or inactivation, and at least 99-percent (2-log) removal of Cryptosporidium. Adequately designed and operated water treatment plants, with coagulation, flocculation, sedimentation and filtration are assigned a 2.5-log removal credit for Giardia, leaving only 0.5-log inactivation to be achieved by …
Removal of Inorganics
- Coagulation operations can be useful in some cases for the removal of inorganics. Examples of successful applications are copper and mercury reductions from wastewaterplant effluents. Two applications discussed in more detail below are arsenic and fluoride removals in potable water treatment:
Wastewater Treatment
- Physical-chemical treatment of wastewater was widely practiced until the late 19th century, until the advent of the trickling filter for biological treatment. The early 1970s saw a partial revival of interest that has continued to the present day, particularly for treatment plants that are overloaded during peak flow events. The addition of coagulant chemicals to primary clarifiers, or to other de…
Factors Affecting Coagulation Operations
- Temperature
Temperature significantly affects coagulation operations, particularly for low turbidity waters, by shifting the optimum pH. This can be mitigated by operating at an optimum pOH as given by: pH + pOH = pKW; where pKW = 0.01706xT + 4470.99/T – 6.0875 and T = temperature in °K = 273.15 … - Sequence of chemical addition
Traditionally, the sequence of chemical addition for coagulation operations is to first add chemicals for pH correction, then add the metal coagulant, then add the flocculant aid. Not all these chemicals are necessarily added, but the sequence logic is often as described. However, t…
Rapid Mixing
- The rapid mixing stage is possibly the most important component of coagulation-flocculation processes, since it is here that destabilization reactions occur and where primary floc particles are formed, whose characteristics markedly influence subsequent flocculation kinetics. In general it is likely that the metal coagulant hydrolysis products that are formed within the tim…
Flocculation
- Orthokinetic flocculation arises from induced velocity gradients in the liquid. It is here that primary particles are induced to approach close enough together, make contact and progressively form larger agglomerates, or flocs. The principal parameter governing the rate of orthokinetic flocculation is the velocity gradient applied. The degree or extent of flocculation is g…
Testing and Control
- The efficiency of the coagulation-flocculation process is dependent on many variables. For a particular water these may include: 1. Type of coagulant used 2. Coagulant dosage 3. Final pH 4. Coagulant feed concentration 5. Type and dosage of chemical additives other than primary coagulant (e.g. polymers) 6. Sequence of chemical addition and time lag between dosing points …