
What happens to chlorine in a water treatment plant?
For drinking water, the water providers add chlorine (Cl2) or hypochlorite to the water at the treatment plant. There, they pressurize the chlorine gas to convert it to a liquid. When it dissolves in the water, the chlorine converts to hypochlorous and hydrochloric acid (HCl).
Why do they add chlorine to tap water?
Public water treatment plants add chlorine to drinking water to kill germs that cause waterborne illnesses, including cholera and typhoid. Added chlorine that effectively sanitizes contaminants is referred to as free chlorine, meaning that this chlorine is “free” to eliminate harmful microorganisms.
Is chlorine bad for plants?
The Centers for Disease Control and Prevention tells us plants are not harmed by water treated with chlorine. Most of us have been watering our plants with chlorinated water for years and they survive. How does Chlorine impact the soil? Chlorine kills some of the microbes in your soil.
How is chlorination used to treat waterborne diseases?
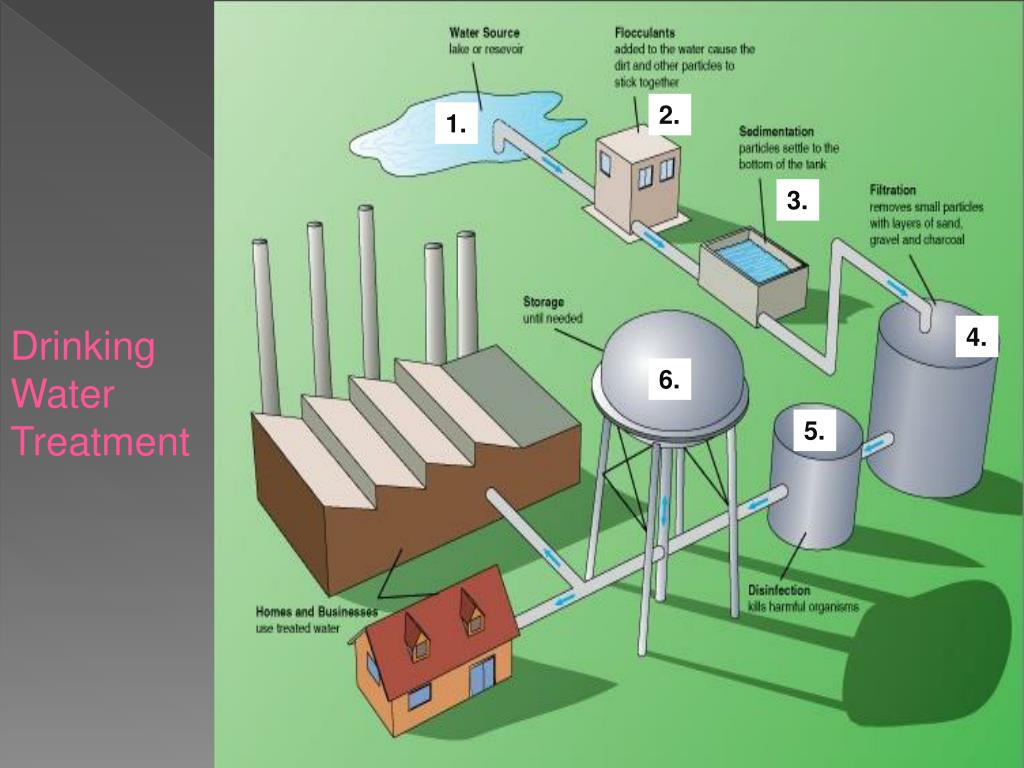
In order to combat waterborne diseases, different disinfection methods are used to inactivate pathogens. Along with other water treatment processes such as coagulation, sedimentation, and filtration, chlorination creates water that is safe for public consumption.
See more
Why is chlorine added to water at water treatment plants?
Chlorination is the process of adding chlorine to drinking water to kill parasites, bacteria, and viruses. Different processes can be used to achieve safe levels of chlorine in drinking water.
Why is chlorine added to water even though it is toxic?
Chlorine effectively kills a large variety of microbial waterborne pathogens, including those that can cause typhoid fever, dysentery, cholera and Legionnaires' disease. Chlorine is widely credited with virtually eliminating outbreaks of waterborne disease in the United States and other developed countries.
Is chlorine treated water safe to drink?
Is chlorinated water safe to drink? Yes. The U.S. Environmental Protection Agency (EPA) limits the amount of chlorine in drinking water to levels that are safe for human consumption. The levels of chlorine used for drinking water disinfection are unlikely to cause long-term health effects.
What happens when chlorine is added to water?
Chlorine will react in water to form hypochlorous acid, which can then dissociate into hydrogen and hypochlorite ions, according to Eqn (1). This reaction is very important, as the disinfecting power of HOCl, hypochlorous acid, is about 40–80 times that of OCl−, hypochlorite.
What is the purpose of adding chlorine to water?
The main objective of this chlorine addition is to disinfect the water and maintain chlorine residuals that will remain in the water as it travels through the distribution system.
Why is chlorination important in water treatment?
In order to combat waterborne diseases, different disinfection methods are used to inactivate pathogens. Along with other water treatment processes such as coagulation, sedimentation, and filtration, chlorination creates water that is safe for public consumption.
What is the combination of free chorine and hypochlorite?
At lower pH levels, the hypochlorous acid will dominate. The combination of hypochlorous acid and hypochlorite ions makes up what is called ‘free chorine.’. Free chlorine has a high oxidation potential and is a more effective disinfectant than other forms of chlorine, such as chloramines.
What is chlorine breakpoint?
Residual Chlorine, Breakpoint. Any type of chlorine that is added to water during the treatment process will result in the formation of hypochlorous acid (HOCl) and hypochlorite ions (OCl-), which are the main disinfecting compounds in chlorinated water. More detail is provided later on in this fact sheet.
How is calcium hypochlorite made?
Calcium hypochlorite (CaOCl) is made up of the calcium salts of hypochlorous acid. It is produced by dissolving chlorine gas (Cl 2) into a solution of calcium oxide (CaO) and sodium hydroxide (NaOH). Calcium hypochlorite is a white, corrosive solid that comes either in tablet form or as a granular powder. Calcium hypochlorite is very stable, and when packaged properly, large amounts can be purchased and stored until needed. The chemical is very corrosive however, and thus requires proper handling when being used to treat water. Calcium hypochlorite needs to be stored in a dry area and kept away from organic materials. It cannot be stored near wood, cloth or petrol because the combination of calcium hypochlorite and organic material can create enough heat for an explosion. It must also be kept away from moisture because the tablets/granular powder readily adsorb moisture and will form (toxic) chlorine gas as a result. Calcium hypochlorite has a very strong chlorine odour – something that should be kept in mind when placing them in storage.
What happens after chlorine demand is met?
After the breakpoint, any additional chlorine added will result in a free chlorine residual proportional to the amount of chlorine added.
How much calcium hypochlorite is needed for water treatment?
Compared to the 1-16 mg/L required with chlorine gas, only 0.5-5 mg/L of calcium hypochlorite is required. When calcium hypochlorite is added to water, hypochlorite and calcium ions are produced.
How does chlorine affect soil?
How does Chlorine impact the soil? Chlorine kills some of the microbes in your soil. Making worm tea with chlorinated water defeats the purpose of worm tea. Colorado’s state extension service tells us chlorinated drinking water may kill a number of microorganisms in soil or a compost pile.
How long does chlorinated water stay in soil?
In one study, researchers continuously applied highly chlorinated water to soil for 126 days. Two days after they stopped, the soil microorganism populations reached pre-treatment levels at all depths of soil.
What is chlorine used for?
It is produced commercially by electrolysis of sodium chloride brine and used as a disinfectant and found in many household cleaning products. It is the basis for most common bleaches.
Why do plants look better after rain?
There are a few reasons why your garden looks better after a rain. One of these reasons is your plants are receiving non-chlorinated water. I noticed that most indoor agricultural growing facilities using city water run the water through reverse osmosis units to remove chlorine from their water.
Does chlorine kill germs?
This is actually great news because the chlorine or chloramine kills disease-causing germs like salmonella and norovirus. However, when I was shelling out money ...
Can plants survive chlorinated water?
The Centers for Disease Control and Prevention tells us plants are not harmed by water treated with chlorine. Most of us have been watering our plants with chlorinated water for years and they survive.
Does carbon filter remove chlorine?
The carbon filters remove the chlorine. I would also guess anyone making beer or bread/pizza (yeast in water) would want to be sure to eliminate chlorine from their water as well. The next time you water your garden is a good time to think about the chemicals in your water.
What is chlorine dioxide?
It is a chemical compound in a gaseous form possessing antimicrobial properties, which is used to disinfect water, surfaces, and a host of other areas.
Why use chlorine dioxide solution in water treatment?
The water reaching our taps is removed of the most harmful pathogens in the water treatment plants using chlorine dioxide. When reaching such plants the water contains pollutants such as parasites, chemicals, bacteria, or even human waste.
Chlorine dioxide to safeguard cooling water systems
Cooling water systems are used as heat sink in industries to remove heat generated through industrial processes. If the heat is not removed the equipment used in industries can get damaged. However, the capacity of such heat sinks can suffer due to the formation of biofilm inside.
Why is chlorine added to water?
When chlorine is added to your water supply, it rapidly reduces the spread of all kinds of waterborne diseases, like cholera and typhoid fever, as well as other ailments. It also makes it easier for cities and towns to purify drinking water to keep residents (like yourself) safe.
How to treat chlorinated water?
Our recommended approach to treating chlorinated water is filtration. By running the water through a filter with activated charcoal in granular or particle form, you can significantly reduce the chlorine and chloramine contents in your water, as well as the general taste and odor associated with chlorine and DBPs.
Why is chlorine bad for you?
The high toxicity of chlorine makes it a powerful chemical that can destroy bacteria, microbes, and pathogens that can leach into your water supply. By killing these disease-causing germs, the compound helps to make water safe to drink. Waterborne diseases have killed thousands of U.S. residents every year.
Why is chlorine used in disinfecting water?
for years, mainly because of its cost-effectiveness, ease of use, wide-scale availability, and proficiency at destroying most pathogens that cause some of the most dangerous waterborne illnesses today.
What is the name of the chemical that is added to pool water?
Instead, liquid chlorine or sodium hypochlorite (NaOCl) is added to the pool water. When either of these forms of chlorine is pumped into the water, it creates hypochlorous acid (HOCl), which is highly active against all bacterial, viral, and fungal human pathogens.
How to find out how much chlorine is in water?
The fastest way to find out is to either request a water quality report from your local municipality or purchase a DIY home water test kit and check your water for chlorine.
What is the most common disinfectant used in water?
Chemical purification. Chlorine is by far the most commonly used water disinfectant worldwide. Today, about 98% of U.S. municipalities use some chlorine- related process to treat their drinking water, thanks to the chemical’s wide-scale availability, low cost, ease of use, and proficiency at destroying germs.
What are the challenges of sodium hypochlorite?
Sodium hypochlorite poses three challenges your water treatment facility will need to overcome: UV rays, off-gassing, and oxidation. Poly Processing has established a set of best practices and containment solutions that overcome these challenges.
Is chlorine gas dangerous?
Chlorine gas is incredibly dangerous. If there’s a leak near residential or school zones, there could be a significant kill zone.
Is sodium hypochlorite an oxidizer?
Sodium hypochlorite is an incredibly aggressive oxidizing chemical. Using an antioxidant barrier can more than double the life of the chemical storage tank system and help ensure the safety of the tank. Traditional polyethylene tanks may fail when storing an oxidizer, but an XLPE tank with an inner-surface engineered polyethylene system helps protect your investment long term.