
Who pays for Regeneron treatment?
The drug, a type of monoclonal antibody, is given by intravenous infusion and costs $1,250 per dose. The Trump administration will pay Eli Lilly $375 million to supply 300,000 doses of its experimental antibody drug to treat COVID-19, the Department of Health and Human Services said Wednesday.
How expensive is monoclonal antibody therapy?
The monoclonal drug is expensive but the federal government is covering the cost. “The drug itself is provided free to the sites. That is significant because the drug normally costs between $3,000 to $5,000 a dose,” Dr. Michael Saag, UAB Infectious Diseases, said. Still you can expect to pay other costs associated with the treatment.
How expensive is monoclonal antibodies?
Monoclonal antibody treatments will be offered at no cost to patients who receive this care. Monoclonal antibody treatment for COVID-19 is available by appointment only. At this time, mAB infusion is reserved for patients over 65 or those with certain high ...
Does insurance pay for monoclonal antibodies?
Medicare Part B (Medical Insurance) covers a COVID-19 monoclonal antibody treatment, if all of these apply: You tested positive for COVID-19. You have a mild to moderate case of COVID-19. You’re at high risk of progressing to a severe case of COVID-19 and/or at high risk of requiring hospitalization.
COVID-19 VEKLURYTM (remdesivir)
Following the recent statement from the National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel about therapies for the COVID-19 Omicron variant, CMS created HCPCS code J0248 for VEKLURY™ (remdesivir) antiviral medication when administered in an outpatient setting.
COVID-19 Monoclonal Antibody Products
The FDA authorized the following investigational monoclonal antibody product under EUA for pre-exposure prophylaxis of COVID-19:
Important Update about Viral Variants
On April 16, 2021, the FDA revoked the EUA for bamlanivimab, when administered alone , due to a sustained increase in COVID-19 viral variants in the U.S. that are resistant to the solo product.
Medicare Coverage for COVID-19 Monoclonal Antibody Products
During the COVID-19 public health emergency (PHE), Medicare will cover and pay for these infusions (when furnished consistent with their respective EUAs) the same way it covers and pays for COVID-19 vaccines.
Coding for the Administration of COVID-19 Monoclonal Antibody Products
CMS identified specific code (s) for each COVID-19 monoclonal antibody product and specific administration code (s) for Medicare payment:
Medicare Payment for Administering COVID-19 Monoclonal Antibody Products
To ensure immediate access during the COVID-19 PHE, Medicare covers and pays for these infusions and injections in accordance with Section 3713 of the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) .
Billing for Administering COVID-19 Monoclonal Antibody Products
Health care providers can bill on a single claim for administering COVID-19 monoclonal antibody products, or submit claims on a roster bill.
How is monoclonal antibody therapy administered?
Dr. Huang: Monoclonal antibody therapy is given through intravenous (IV) infusion. These infusions are given in one of our outpatient infusion centers and require about an hour to administer, followed by an hour of observation and monitoring.
What is the purpose of monoclonal antibody therapy?
The goal of this therapy is to help prevent hospitalizations, reduce viral loads and lessen symptom severity.
Can I receive monoclonal antibody therapy if I'm pregnant or breastfeeding?
Dr. Huang: Because there's very limited data regarding how this therapy affects pregnant women and unborn babies, the risk of this new therapy may outweigh the benefits in some cases. If you are high risk and develop COVID-19 while pregnant or breastfeeding, it's important to discuss your treatment options and your specific situation with your doctor.
Is there anything I need to know about receiving monoclonal antibody therapy?
Dr. Huang: After receiving monoclonal antibody therapy, it's recommended that you wait 90 days before receiving the COVID-19 vaccine. If you already received the first dose of vaccine before monoclonal antibody therapy, current CDC guidelines recommend you wait 90 days before receiving the second dose.
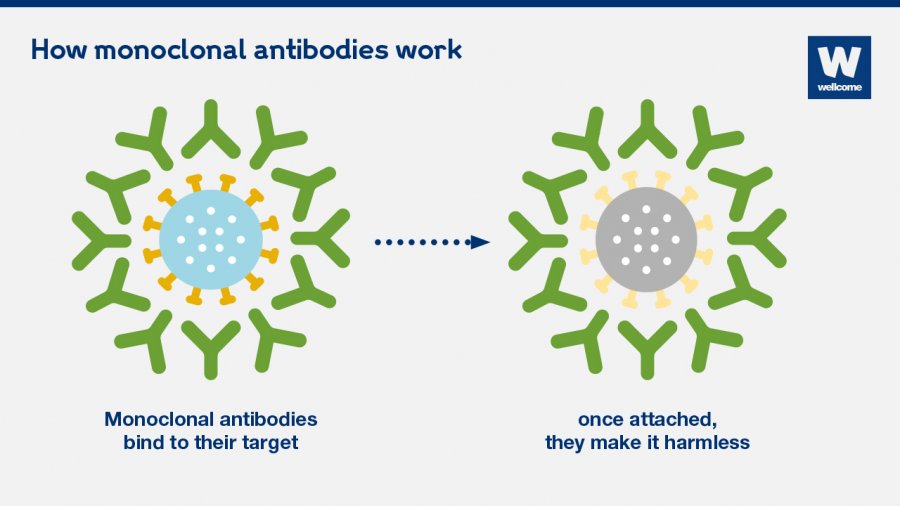
What type of antibody is used to recognize a specific component of a virus?
This type of therapy relies on monoclonal antibodies. These are antibodies that are similar to the ones your body would naturally make in response to infection. However, monoclonal antibodies are mass-produced in a laboratory and are designed to recognize a specific component of this virus — the spike protein on its outer shell.
What antibodies interfere with the virus?
By targeting the spike protein, these specific antibodies interfere with the virus' ability to attach and gain entry into human cells. The two monoclonal antibody therapies currently available are the bamlanivimab and a combination of the casirivimab and imdevimab.
Can monoclonal antibodies cause allergic reactions?
One possible side effect of monoclonal antibody therapy is an allergic reaction. These reactions typically only occur during infusion or soon after, and your care team will closely monitor for any signs of an allergic reaction. However, because an infusion reaction can also be delayed, contact your doctor immediately if you notice any of the following signs of an allergic reaction:
Overview
Monoclonal antibodies (also called moAbs or mAbs) are proteins made in laboratories that act like proteins called antibodies in our bodies. Antibodies are parts of your immune system. They seek out the antigens (foreign materials) and stick to them in order to destroy them.
Procedure Details
In most cases, monoclonal antibodies are given mostly as intravenous (IV) solution injected right into your vein (sometimes referred to as an infusion). They’re often given in an infusion center where there are several people getting treatment at one time.
Recovery and Outlook
Infusion times can vary. As an example, though, monoclonal antibody treatment for COVID-19 under Emergency Use Authorization took about an hour for infusion and then another hour or so to watch for any reaction to the infusion.
When to Call the Doctor
If you’ve had a monoclonal antibody treatment, and you’re having an expected reaction, call your healthcare provider or go to an emergency room.
What is monoclonal antibody?
Monoclonal antibodies are laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens such as viruses. You naturally make antibodies to fight infections, but your body may not have antibodies designed to recognize a novel (or new) virus like SARS-CoV-2, the virus that causes COVID-19. Unlike the COVID-19 vaccine, which can take two doses and weeks to help your body develop enough antibodies to prevent an infection, monoclonal antibody treatments quickly give your body the antibodies it needs to protect against an active infection.
Can you get a fever after an antibody infusion?
These infusions can help patients who have been diagnosed with COVID-19 and are at risk of developing a severe case of the illness. Early clinical trials have shown that this treatment may reduce the need for hospitalization and severe illness. Allergic reactions can happen during and after an antibody infusion, including fever, chills, nausea, headache, shortness of breath, low blood pressure, wheezing, swelling or a rash, itching, muscle aches, or dizziness. Other risks to consider are that the antibody infusion can interfere with your body’s ability to fight off a future infection of COVID-19 or may reduce your body’s immune response to the COVID-19 vaccine.
