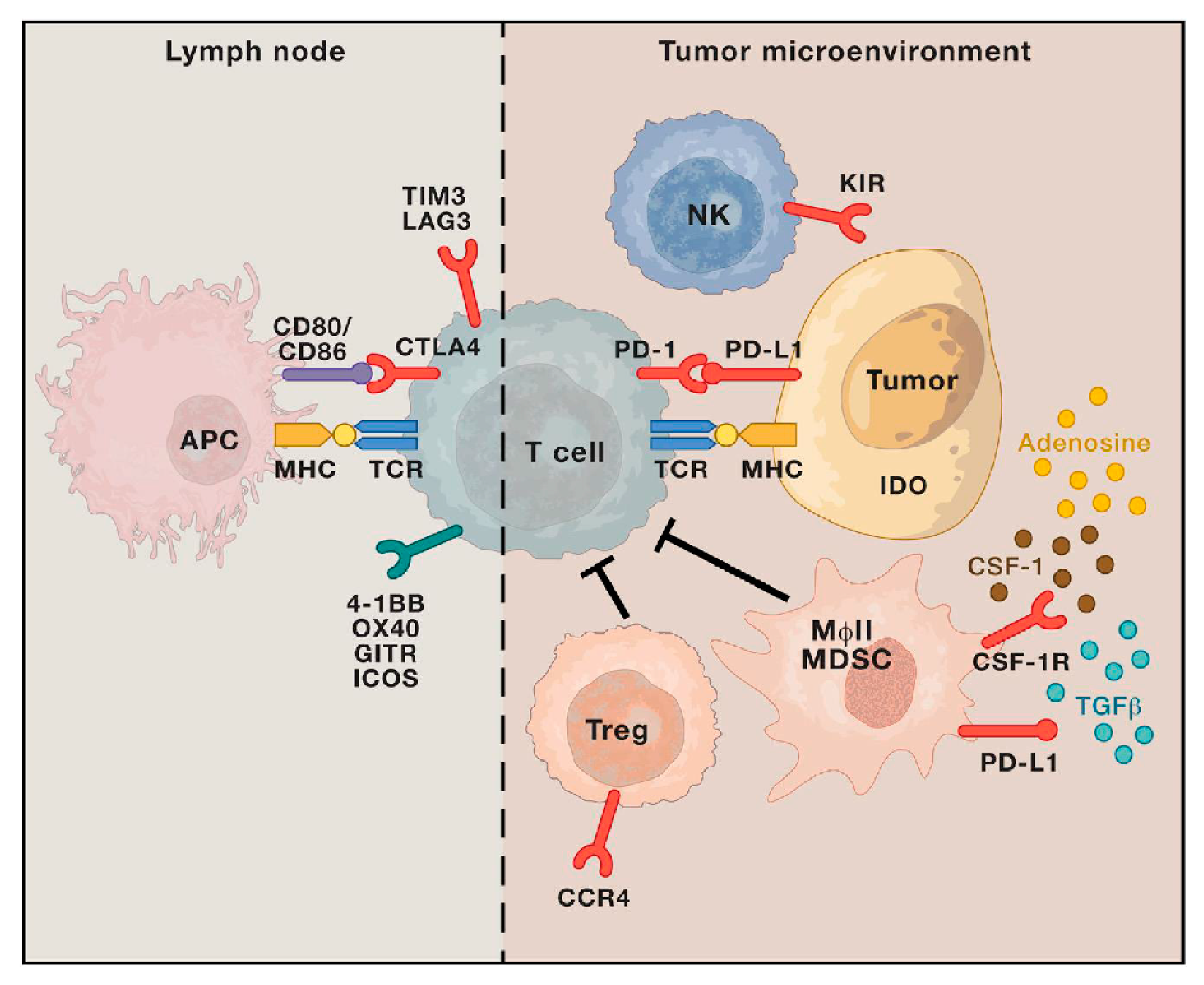
Who can get Regeneron treatment?
Jan 06, 2022 · Who is eligible for monoclonal antibody therapy? Given that COVID-19 vaccination provides strong protection against severe disease and need for hospitalization, monoclonal antibody therapy is an option for certain high-risk patients with COVID-19. THE FDA expanded EUA of two monoclonal antibody treatments to include patients as young as newborns. Criteria for …
What are the dangers of monoclonal antibodies?
How is monoclonal antibody or antiviral treatment done? At this time, UCHealth uses Sotrovimab, which is available by FDA Emergency Use Authorization. Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous (IV) infusion. Another option for COVID-19 therapy is an antiviral called Remdesivir.
How effective is the monoclonal treatment?
Tocilizumab (EUA issued June, 24 2021) Bebtelovimab (EUA issued February 11, 2022) The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply: The patient has a …
How often can you get monoclonal antibodies?
To receive a mAb you should be referred for treatment by your healthcare professional and directed to available infusion locations. If you do not have a healthcare provider, call the Combat COVID Monoclonal Antibodies Call Center at 1-877-332-6585 to find out who to talk with about your symptoms and treatment.
Who could benefit from monoclonal antibody therapy to prevent COVID-19?
See full answerVaccines are the best way to protect against COVID-19. But some people with weakened immune systems do not produce enough antibodies after vaccination, and others are severely allergic to the vaccine. The FDA recently authorized Evusheld, a pre-exposure prophylaxis (PrEP) monoclonal antibody therapy developed by AstraZeneca, which should help prevent COVID-19 in these populations.To be eligible for Evusheld, individuals must be 12 years or older and have a moderately to severely weakened immune system, or have a history of severe adverse reactions to the COVID-19 vaccine or its components. In addition, the therapy cannot be given to someone with a current SARS-CoV-2 infection, or who has been recently exposed to someone who is infected. Evusheld is given as two consecutive shots, and evidence suggests it can help prevent symptomatic infection for at least six months.Apr 1, 2022
What is a monoclonal antibody for COVID-19?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells. Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
Is there a monoclonal antibody therapy for post COVID-19 exposure?
FDA authorizes bamlanivimab and etesevimab monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19 | FDA.Sep 16, 2021
Can I get the COVID-19 vaccine if I was treated with monoclonal antibodies or convalescent plasma?
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
What is a monoclonal antibody?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells.Mar 31, 2022
What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
Is there an antibody cocktail for COVID-19?
The treatment, bamlanivimab and etesevimab administered together, was granted FDA emergency use authorization in February. Eli Lilly and the FDA stipulated that the antibody cocktail is authorized as a COVID-19 prophylaxis only for individuals who have been exposed to the virus.Sep 16, 2021
Is it possible to develop immunity to COVID-19 after being exposed?
In addition, the hope is that people who've been exposed to COVID-19 also develop an immunity to it. When you have immunity, your body can recognize and fight off the virus. It's possible that people who've had COVID-19 can get sick again -- and maybe infect other people.Jan 21, 2022
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
What medication is not recommended before vaccinations for COVID-19?
It is not recommended you take over-the-counter medicine – such as ibuprofen, aspirin, or acetaminophen – before vaccination for the purpose of trying to prevent vaccine-related side effects. It is not known how these medications might affect how well the vaccine works.
Who should not take the Pfizer-BioNTech COVID-19 vaccine?
If you have had a severe allergic reaction to any ingredient in the Pfizer-BioNTech COVID-19 vaccine (such as polyethylene glycol), you should not get this vaccine. If you had a severe allergic reaction after getting a dose of the Pfizer-BioNTech COVID-19 vaccine, you should not get another dose of an mRNA vaccine.