What happens during the lead discovery process?
During the lead discovery, phase molecules are also screened in cell-based assays predictive of the disease state and in animal models of disease to characterize both the efficacy of the compound and its likely safety profile (Figure 2).
What is lead optimization in drug discovery?
Lead optimization phase The object of this final drug discovery phase is to maintain favourable properties in lead compounds while improving on deficiencies in the lead structure. Continuing with example above, the aim of the programme was now to modify the structure to minimize hERG liability and to improve the absorption of the compound.
What are the treatment options for lead toxicity?
If lead ingestion is suspected, an abdominal x-ray and potential bowel decontamination should be performed. A BLL ≥45 mcg/dL requires the initiation of outpatient chelation therapy, and a BLL ≥70 mcg/dL requires hospitalization and chelation therapy. 6
What should be included in the investigation of lead toxicity?
An environmental investigation is needed, and steps to reduce lead exposure should be taken. The patient should be monitored for neurodevelopmental changes and iron status. If lead ingestion is suspected, an abdominal x-ray and potential bowel decontamination should be performed.

When was lead poison discovered?
Doctors have recognized that high doses of lead are downright poisonous since, at least, the days of Hippocrates. But it was not until March 29, 1979 that a pediatrician and child psychiatrist named Herbert Needleman first documented the dangers of even the lowest forms of lead exposure.
What is the antidote for lead poisoning?
There is no antidote for lead. Treatment of lead poisoning consists of removal from the source of exposure. Chelation therapy should be considered for treatment of severe symptoms or markedly elevated blood lead levels.
Why lead poisoning is called Plumbism?
Lead poisoning, also known as plumbism and saturnism, is a type of metal poisoning caused by lead in the body. The brain is the most sensitive. Symptoms may include abdominal pain, constipation, headaches, irritability, memory problems, infertility, and tingling in the hands and feet....Lead poisoningSpecialtyToxicology13 more rows
Where does lead originate?
Lead can be found in all parts of our environment – the air, the soil, the water, and even inside our homes. Much of our exposure comes from human activities including the use of fossil fuels including past use of leaded gasoline, some types of industrial facilities and past use of lead-based paint in homes.
What is EDTA antidote?
Ethylenediaminetetraacetic acid (EDTA) is a medication used in the management and treatment of heavy metal toxicity. It is in the chelating class of drugs.
How does EDTA treat lead poisoning?
Lead poisoning and heavy metal toxicity Chelation therapy using EDTA is the medically-accepted treatment for lead poisoning. Injected intravenously and once in the bloodstream, EDTA traps lead and other metals, forming a compound that the body can eliminate in the urine. The process generally takes 1 to 3 hours.
Who discovered lead?
Ancient Egyptians were likely the first to extract lead, which they used to make small sculptures. Compounds of lead have also been found in Egyptian pottery glazes. In China, lead was used to forge coins by 2000BC.
What is plumb ism?
Definition of plumbism : lead poisoning especially when chronic.
Why is lead poisoning called saturnism?
The ancient name for lead poisoning, saturnism, derives from the alchemic name for lead. Although now much less relevant, this malign influence of Saturn is still here and can only be kept at bay by continued vigilance. This is especially true in times of potential exploitation of lowly paid workers.
How was lead named?
Lead is chiefly obtained from the mineral galena by a roasting process. At least 40% of lead in the UK is recycled from secondary sources such as scrap batteries and pipes....Discovery dateAncientOrigin of the nameThe name comes from the Anglo-Saxon word for the metal, 'lead'Allotropes1 more row
Who discovered bismuth?
Claude François GeoffroyBismuth / DiscovererThe element bismuth was officially discovered in 1753 by Claude Geoffrey Junine, but has been utilized since the Middle Ages and in Ancient Egypt. It is a hard and brittle metallic element found in Group 15 of the periodic table.
When was tin first discovered?
Tin was first used in 3500 BC in the city of Ur in southern Mesopotamia, now known as Iraq. The natives of Iran made articles from bronze, which is an alloy of tin and copper. The earliest uses of tin were in Turkey. It was first mined and refined in Turkey.
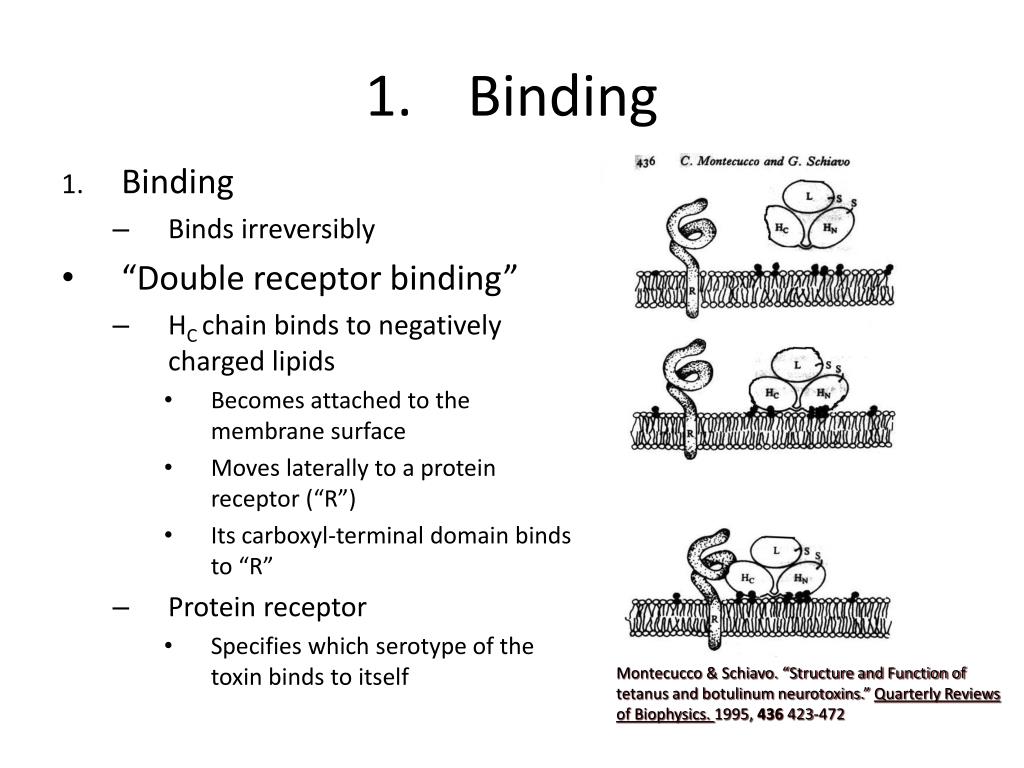
How does binding work in lead?
Binding is usually gained during lead optimization by increasing lipophilicity ( this is a simple way to gain binding), which in turn decreases aqueous solubility and hence drug disposition. In the past, one first optimized potency and selectivity. The ADMET properties were addressed at a later stage.
What is lead compound?
Lead compound is a chemical compound or natural product that has biological activity against a drug target. Lead identification and optimization is a crucial step in the drug discovery program. Chemical compounds or natural products could be the best source or starting point to obtain potential lead/s with improved biological activity, selectivity, ...
Why is it important to develop a blueprint for drug discovery?
Because many drug candidates are sparingly soluble, it is often necessary to devise a dosage form even in the early phase of drug discovery .
How do humans get exposed to lead?
Humans are most often exposed to lead through inhalation and ingestion, though inhaling lead results in a larger percentage of lead entering into the blood stream [19]. While lead compounds do occur naturally, most of the lead-related environmental exposures come from anthropogenic sources [58,59].
Why are children at risk for lead exposure?
Children, particularly, are at risk for lead exposure due to an increased propensity to ingest dust or soil containing lead [68]. Furthermore, children show a higher relative gastrointestinal absorption of lead [69].
Why is lead used in gasoline?
In the past, lead was used in gasoline in order to improve octane ratings and to increase engine performance [ 63]. The United States required the removal of lead from gasoline in 1986, as a consequence of the introduction of the catalytic converter and of public health concerns about exposure to lead [63].
Which civilizations mined lead?
Civilizations ranging from the Romans to ancient Indians mined, refined, and used lead [61,62]. Lead compounds continue to be produced in vast quantities (millions of pounds) annually in the United States [19,59].
Where did lead mining occur?
The largest production of lead occurred in South and East Asia, especially China and India, where lead mining grew rapidly.
Where is lead found?
World lead resources exceed two billion tons. Significant deposits are located in Australia, China, Ireland, Mexico, Peru, Portugal, Russia, and the United States.
What are the two oxidation states of lead?
Lead shows two main oxidation states: +4 and +2. The tetravalent state is common for the carbon group. The divalent state is rare for carbon and silicon, minor for germanium, important (but not prevailing) for tin, and is the more important of the two oxidation states for lead. This is attributable to relativistic effects, specifically the inert pair effect, which manifests itself when there is a large difference in electronegativity between lead and oxide, halide, or nitride anions, leading to a significant partial positive charge on lead. The result is a stronger contraction of the lead 6s orbital than is the case for the 6p orbital, making it rather inert in ionic compounds. The inert pair effect is less applicable to compounds in which lead forms covalent bonds with elements of similar electronegativity, such as carbon in organolead compounds. In these, the 6s and 6p orbitals remain similarly sized and sp 3 hybridization is still energetically favorable. Lead, like carbon, is predominantly tetravalent in such compounds.
How does lead form a chain?
Lead can form multiply-bonded chains, a property it shares with its lighter homologs in the carbon group. Its capacity to do so is much less because the Pb–Pb bond energy is over three and a half times lower than that of the C–C bond. With itself, lead can build metal–metal bonds of an order up to three. With carbon, lead forms organolead compounds similar to, but generally less stable than, typical organic compounds (due to the Pb–C bond being rather weak). This makes the organometallic chemistry of lead far less wide-ranging than that of tin. Lead predominantly forms organolead (IV) compounds, even when starting with inorganic lead (II) reactants; very few organolead (II) compounds are known. The most well-characterized exceptions are Pb [CH (SiMe 3) 2] 2 and Pb ( η5 -C 5 H 5) 2.
How much tensile strength does lead have?
In comparison, that of aluminium is 75.2 GPa; copper 137.8 GPa; and mild steel 160–169 GPa. Lead's tensile strength, at 12–17 MPa, is low (that of aluminium is 6 times higher, copper 10 times, and mild steel 15 times higher); it can be strengthened by adding small amounts of copper or antimony.
What color is lead when it is cut?
When freshly cut, lead is silvery with a hint of blue; it tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is a relatively unreactive post-transition metal.
How many electrons does lead have?
Atomic. A lead atom has 82 electrons, arranged in an electron configuration of [ Xe ]4f 14 5d 10 6s 2 6p 2. The sum of lead's first and second ionization energies —the total energy required to remove the two 6p electrons—is close to that of tin, lead's upper neighbor in the carbon group.
Why do drug discovery assays fail?
Target identification. Drugs fail in the clinic for two main reasons; the first is that they do not work and the second is that they are not safe. As such, one of the most important steps in developing a new drug is target identification and validation.
What is tissue based drug?
A tissue-based approach fordetermination of the effects of a drug at the tissue rather than the cellular or subcellular level, for example, muscle contractility. Bespoke screens of lower throughput. Aim to more closely mimic the complexity of tissue rather than just looking at single readouts.
What is hit molecule?
A ‘hit’ molecule can vary in meaning to different researchers but in this in review we define a hit as being a compound which has the desired activity in a compound screen and whose activity is confirmed upon retesting. A variety of screening paradigms exist to identify hit molecules (see Table 1).
What are some examples of mutations in the amyloid precursor protein?
For example, familial Alzheimer's Disease (AD) patients commonly have mutations in the amyloid precursor protein or presenilin genes which lead to the production and deposition in the brain of increased amounts of the Abeta peptide, characteristic of AD (Bertram and Tanzi, 2008).
What was the name of the drug that Alexander Fleming discovered?
Alexander Fleming’s Discovery of Penicillin. Penicillin heralded the dawn of the antibiotic age. Before its introduction there was no effective treatment for infections such as pneumonia, gonorrhea or rheumatic fever. Hospitals were full of people with blood poisoning contracted from a cut or a scratch, and doctors could do little for them ...
When did Fleming publish his findings?
Fleming published his findings in the British Journal of Experimental Pathology in June 1929, with only a passing reference to penicillin's potential therapeutic benefits.
What happened to the man who snatched the side of his mouth while pruning roses?
He had scratched the side of his mouth while pruning roses, and had developed a life-threatening infection with huge abscesses affecting his eyes, face, and lungs. Penicillin was injected and within days he made a remarkable recovery. But supplies of the drug ran out and he died a few days later.
What did Fleming find?
Fleming found that his "mold juice" was capable of killing a wide range of harmful bacteria, such as streptococcus, meningococcus and the diphtheria bacillus. He then set his assistants, Stuart Craddock and Frederick Ridley, the difficult task of isolating pure penicillin from the mold juice.
What did Fleming find in his petri dishes?
Returning from holiday on September 3, 1928, Fleming began to sort through petri dishes containing colonies of Staphylococcus, bacteria that cause boils, sore throats and abscesses. He noticed something unusual on one dish. It was dotted with colonies, save for one area where a blob of mold was growing.
How many units of morphine were made in 1944?
Production of the drug in the United States jumped from 21 billion units in 1943, to 1,663 billion units in 1944, to more than 6.8 trillion units in 1945, and manufacturing techniques had changed in scale and sophistication from one-liter flasks with less than 1% yield to 10,000-gallon tanks at 80-90% yield.
When did penicillin become available to the public?
In the United Kingdom, penicillin first went on sale to the general public, as a prescription only drug, on June 1, 1946. In Britain, Chain and Abraham continued to work on the structure of the penicillin molecule, aided by the X-ray crystallographic work of Dorothy Hodgkin, also at Oxford.
Which binds to the S1 pocket?
Dabigatran, like NAPAP binds to S1, S2 and S4 pockets. Benzamidine group on the dabigatran structure binds deep in the S1 pocket, the methylbenzimidazole fits nicely in the hydrophobic S2 pocket and the Ile and Leu at the bottom of the S4 pocket binds to the aromatic group of dabigatran.
What is the purpose of the thrombin cascade?
Thrombin has multiple purposes, but mainly it converts soluble fibrinogen to an insoluble fibrin complex.
What was the first DTI?
The first DTI was actually hirudin, which became more easily available with genetic engineering. It is now available in a recombinant form as lepirudin (Refludan) and desirudin (Revasc, Iprivask). Development of other DTIs followed with the hirudin analog, bivalirudin, and then the small molecular DTIs.
What is a small molecule that inhibits thrombin?
Small molecular direct thrombin inhibitors. Small molecular direct thrombin inhibitors (smDTIs) are non-peptide small molecules that specifically and reversibly inhibit both free and clot-bound thrombin by binding to the active site of the thrombin molecule.
What is a direct thrombin inhibitor?
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways.
Can DTIs inhibit thrombin?
DTIs aren't dependent to cofactors like antithrombin to inhibit thrombin so they can both inhibit free/soluble thrombin as well as fibrin bound thrombin unlike heparins. The inhibition is either irreversible or reversible. Reversible inhibition is often linked to lesser risk of bleeding.
Can a drug bind to both the active site and exosite 1?
Drugs can either bind to both the active site and exosite 1 (bivalent) or just to the active site (univalent) . DTIs inhibit thrombin by two ways; bivalent DTIs block simultaneously the active site and exosite 1 and act as competitive inhibitors of fibrin, while univalent DTIs block only the active site and can therefore both inhibit unbound ...

Overview
Lead is a chemical element with the symbol Pb (from the Latin plumbum) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is silvery with a hint of blue; it tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and …
Physical properties
A lead atom has 82 electrons, arranged in an electron configuration of [Xe]4f 5d 6s 6p . The sum of lead's first and second ionization energies—the total energy required to remove the two 6p electrons—is close to that of tin, lead's upper neighbor in the carbon group. This is unusual; ionization energies generally fall going down a group, as an element's outer electrons become more distant from the nucleus, and more shielded by smaller orbitals.
Chemistry
Bulk lead exposed to moist air forms a protective layer of varying composition. Lead(II) carbonate is a common constituent; the sulfate or chloride may also be present in urban or maritime settings. This layer makes bulk lead effectively chemically inert in the air. Finely powdered lead, as with many metals, is pyrophoric, and burns with a bluish-white flame.
Fluorine reacts with lead at room temperature, forming lead(II) fluoride. The reaction with chlorine is …
Origin and occurrence
Lead's per-particle abundance in the Solar System is 0.121 ppb (parts per billion). This figure is two and a half times higher than that of platinum, eight times more than mercury, and seventeen times more than gold. The amount of lead in the universe is slowly increasing as most heavier atoms (all of which are unstable) gradually decay to lead. The abundance of lead in the Solar System since its formation 4.5 billion years ago has increased by about 0.75%. The solar system abundances …
Etymology
The modern English word lead is of Germanic origin; it comes from the Middle English leed and Old English lēad (with the macron above the "e" signifying that the vowel sound of that letter is long). The Old English word is derived from the hypothetical reconstructed Proto-Germanic *lauda- ('lead'). According to linguistic theory, this word bore descendants in multiple Germanic languages of exactly the same meaning.
History
Metallic lead beads dating back to 7000–6500 BCE have been found in Asia Minor and may represent the first example of metal smelting. At that time lead had few (if any) applications due to its softness and dull appearance. The major reason for the spread of lead production was its association with silver, which may be obtained by burning galena (a common lead mineral). The Ancient Egyptians were the first to use lead minerals in cosmetics, an application that spread to Ancient …
Production
As of 2014, production of lead is increasing worldwide due to its use in lead–acid batteries. There are two major categories of production: primary from mined ores, and secondary from scrap. In 2014, 4.58 million metric tons came from primary production and 5.64 million from secondary production. The top three producers of mined lead concentrate in that year were China, Australia, and the United States. The top three producers of refined lead were China, the United States, an…
Applications
Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. When the pencil originated as a wrapped graphite writing tool, the particular type of graphite used was named plumbago (literally, act for lead or lead mockup).
Lead metal has several useful mechanical properties, including high density, low melting point, ductility, and relative inertness. Many metals are superior to lead in some of these aspects but a…