When to give monoclonal antibody treatment?
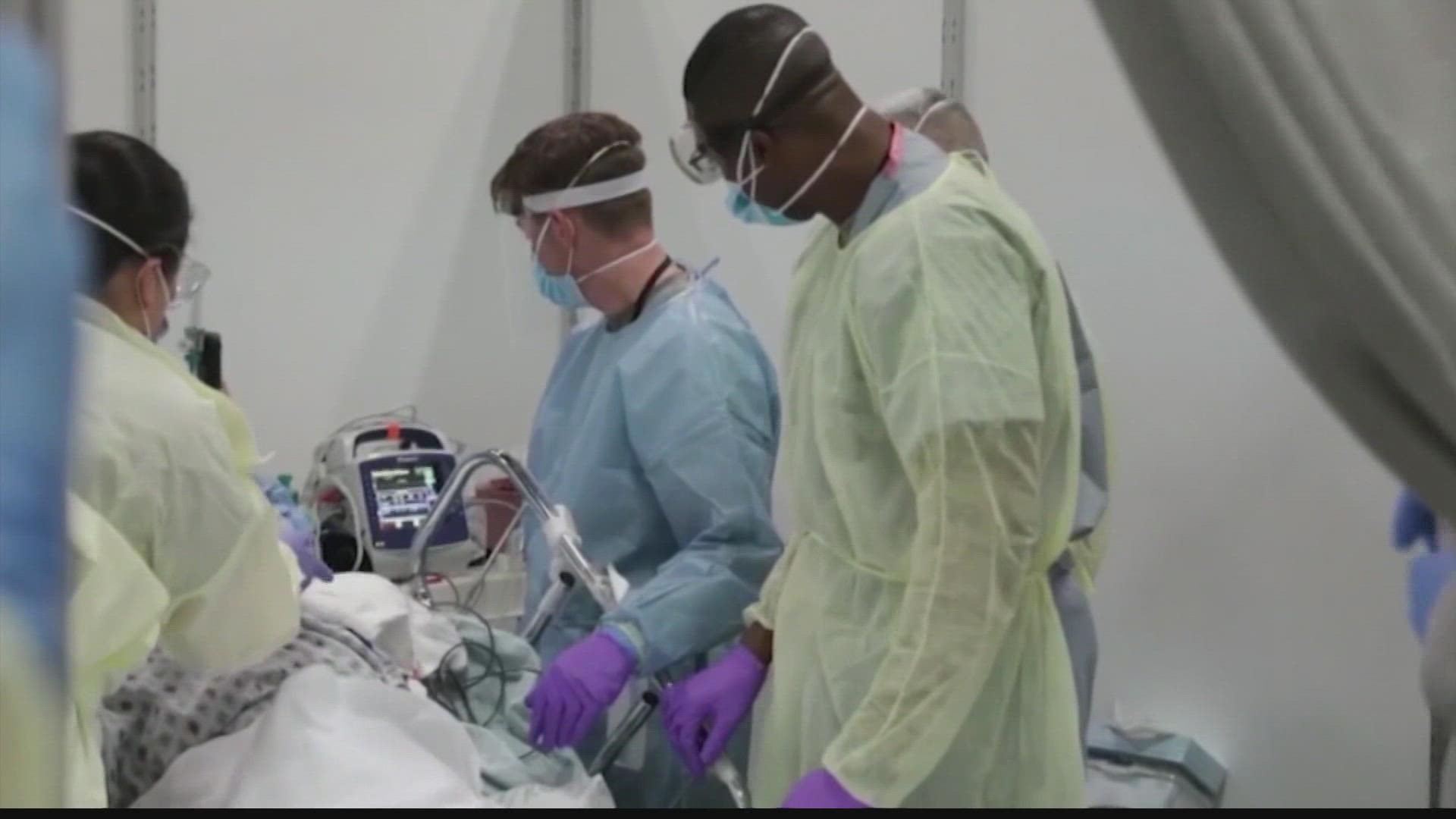
The treatment may protect patients for up to six months. The FDA has authorized AstraZeneca's monoclonal antibody treatment for COVID, designed for patients with weakened immune systems. Pictured: A patient receives Regeneron's monoclonal antibody treatment in Sarasota, Florida, September 2021
What to expect from monoclonal antibody treatment?
- Upset stomach (nausea, vomiting, or diarrhea)
- Itching, swelling, rash, or hives
- Dizziness or low blood pressure
- Changes in your heartbeat
- Any new or worsening symptoms
- Difficulty breathing
- Weakness
- Confusion
Who pays for monoclonal treatment?
- BMI ≥95% for age on CDC growth chart
- Immunosuppressed: congenital or acquired immunodeficiency, solid organ transplant, active hematologic malignancy receiving chemotherapy, bone marrow transplant, or autoimmune diseases requiring immunosuppressive therapy
- Pregnancy
Who is eligible for monoclonal?
Monoclonal antibodies. Monoclonal antibody treatment can be used in people 12 years of age and older who weigh at least 88 pounds (40 kg) who are at high risk for severe COVID-19, including hospitalization or death for: Treatment of mild to moderate symptoms of COVID-19. To be eligible, patients must: Test positive for SARS-CoV-2.
Is there a monoclonal antibody therapy for post COVID-19 exposure?
FDA authorizes bamlanivimab and etesevimab monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19 | FDA.
What is the effectiveness of monoclonal antibody therapy for COVID-19?
Monoclonal antibodies can be effective at decreasing hospitalization rates and progression to severe disease and death for patients with mild to moderate COVID-19. In addition, mAbs have been shown to improve survival in patients hospitalized with COVID-19 who have not mounted their own immune response.
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
What is the latest medication for COVID-19?
Paxlovid is the latest COVID-19 treatment that's been all over the news. The drug was granted an emergency use authorization (EUA) by the Food and Drug Administration (FDA) in December for anyone ages 12 and older who weighs at least 88 pounds, and is at high risk for severe disease.
Are antibodies beneficial during the COVID-19 pandemic?
When reinfections or breakthrough infections happen, having antibodies plays an important role in helping prevent severe illness, hospitalization, and death. For many diseases, including COVID-19, antibodies are expected to decrease or “wane” over time.
Why antibody testing Is not currently recommended to assess immunity after COVID-19 vaccination?
Currently authorized SARS-CoV-2 antibody tests have not been evaluated to assess the level of protection provided by an immune response to COVID-19 vaccination. If antibody test results are interpreted incorrectly, there is a potential risk that people may take fewer precautions against SARS-CoV-2 exposure.
What is the first drug that was approved by the FDA to treat COVID-19?
Remdesivir is the first drug approved by the FDA for treatment of hospitalized COVID patients over the age of 12.
Are there different variants of COVID-19 in the US?
SARS-CoV-2 is constantly changing, and new variants of the virus are expected to occur. In early 2021, the Alpha variant emerged, followed by the Delta variant later that summer. In late 2021 and throughout early 2022, the Omicron variant swept across the country and continues to be the predominant variant circulating in the United States.
How does Remdesivir injection work to treat COVID-19?
Remdesivir is in a class of medications called antivirals. It works by stopping the virus from spreading in the body.
What medication should I take for mild COVID-19 symptoms?
If you are worried about your symptoms, the Coronavirus Self-Checker can assist in the decision to seek care. You can treat symptoms with over-the-counter medicines, such as acetaminophen (Tylenol) or ibuprofen (Motrin, Advil), to help you feel better. Learn more about what to do if you are sick.
What are some of the medications that I can take to reduce the symptoms of COVID-19?
Acetaminophen (Tylenol), ibuprofen (Advil, Motrin) and naproxen (Aleve) can all be used for pain relief from COVID-19 if they are taken in the recommended doses and approved by your doctor.
What is the treatment for mild COVID-19?
Treatment for COVID-19 depends on the severity of the infection. For milder illness, resting at home and taking medicine to reduce fever is often sufficient. Antiviral pills such as Paxlovid or molnupiravir may be prescribed by a doctor if a patient is eligible.
What is monoclonal antibody?
Monoclonal antibodies are lab-created proteins that mimic the ones humans produce to fight viruses and diseases. The antibodies defend the body by blocking Covid-19 viruses from attaching to and entering human cells. The treatment, developed by Regeneron, is shown to reduce the risk of hospitalization or death in 70 percent of patients.
What is the success rate of Regeneron 2021?
At the end of July 2021, Regeneron found that their monoclonal antibody therapy (Regen-CoV) reduced symptomatic infections with an 81% success rate.
When was Regen Cov approved?
The Food and Drug Administration approved Regen-CoV for emergency use in November of 2020. The Regen-CoV treatment has been tested extensively by Regeneron in a clinical trial setting. Regeneron reported 70 percent of patients who took Regen-CoV supported their immune system, leading to the recovery of Covid-19 symptoms.
Does Kaiser insurance cover monoclonal antibody therapy?
At this time theirs is no cost to receive the Monoclonal Antibody Therapy. We accept all insurance plans for this treatment as long as your insurance is active. Including Kaiser Insurance. We will bill your insurance so you may receive the treatment as soon as possible.
Can Regen CoV be distributed in Orange County?
Because of the recent update in Regen-CoV authorization, we can distribute the treatment to more patients that need it in the Orange County area. Book an appointment with one of our healthcare providers today to see if a Regen-CoV treatment plan is right for you.
Can a 12 year old be a monoclonal?
Yes, select children may be eligible for monoclonal antibody therapy. Those who are 12 years of age or older and at high risk for progressing moderate Covid-19 symptoms may be eligible. Schedule a consultation appointment with a healthcare provider to see if Regen-CoV is right for your child.
Monoclonal Antibody Therapy
Glaxo Smith Kline’s monoclonal antibody Sotrovimab is authorized for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in:
Preventative Monoclonal Antibody Therapy: EvuSheld
Preventative monoclonal antibody therapy locations are marked with a blue pin on the locator map.
What is monoclonal antibody therapy?
Monoclonal antibody (mAb) therapy is an injection of lab-made COVID-19 antibodies to help the body fight off the infection.
Monoclonal antibody locations in Colorado
Coloradans can make their own appointment for monoclonal antibody therapy at any state-led site. A prescription is not required, because an on-site provider will determine eligibility.
Find monoclonal antibody therapy in Colorado
Here are other ways to find monoclonal antibody treatments in Colorado.
Treatment Options and Fact Sheets
The following options are available to treat mild-to-moderate symptoms of COVID-19 in people at high-risk for severe disease.
Need help?
Learn more about treatments, eligibility and cost on our Treatments page.
Need a ride?
More transportation resources will be added to this section. Check back for updates.