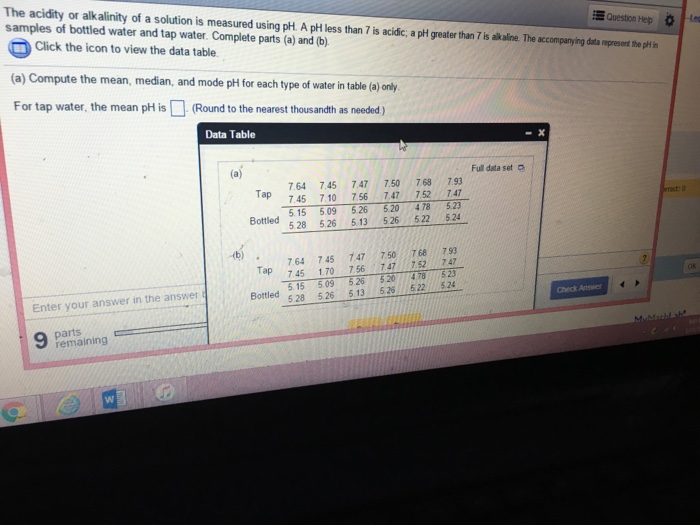
What is the solution for alkalinity in a wastewater treatment plant?
Oct 22, 2019 · One common method the U.S. Geological Survey (USGS) uses for measuring alkalinity is to use take a water sample and to add acid to it while checking the pH of the water as the acid is added. An initial pH reading of the water is taken and then small amounts of acid are added in increments, the water is stirred, and the pH is taken.
What is alkaline treatment or mercerization?
Jan 24, 2022 · Alkalinity of water means acid neutralization capacity of water. When you add acid in water (adding H + ions) water absorbs H + ions without showing significant change in pH. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. The possibility of OH – and HCO 3– ions together is not possible since …
Why alkalinity of water is important in boiler water treatment?
Jan 17, 2022 · Add sodium bisulfate and muriatic acid to lower the alkalinity of the wastewater. How Alkalinity Affects Nitrification Use alkalinity profiling in wastewater operations to control biological activity and optimize process control The Water Environment Federation’s new Operations Challenge laboratory event will determine alkalinity needs to facilitate nitrification.
What is alkalinity?
Feb 17, 2022 · Conventional treatments will remove a variety of secondary contaminants. Coagulation (or flocculation) and filtration removes metals like iron, manganese and zinc. Aeration removes odors, iron, and manganese. Granular activated carbon will remove most of the contaminants which cause odors, color, and foaming.
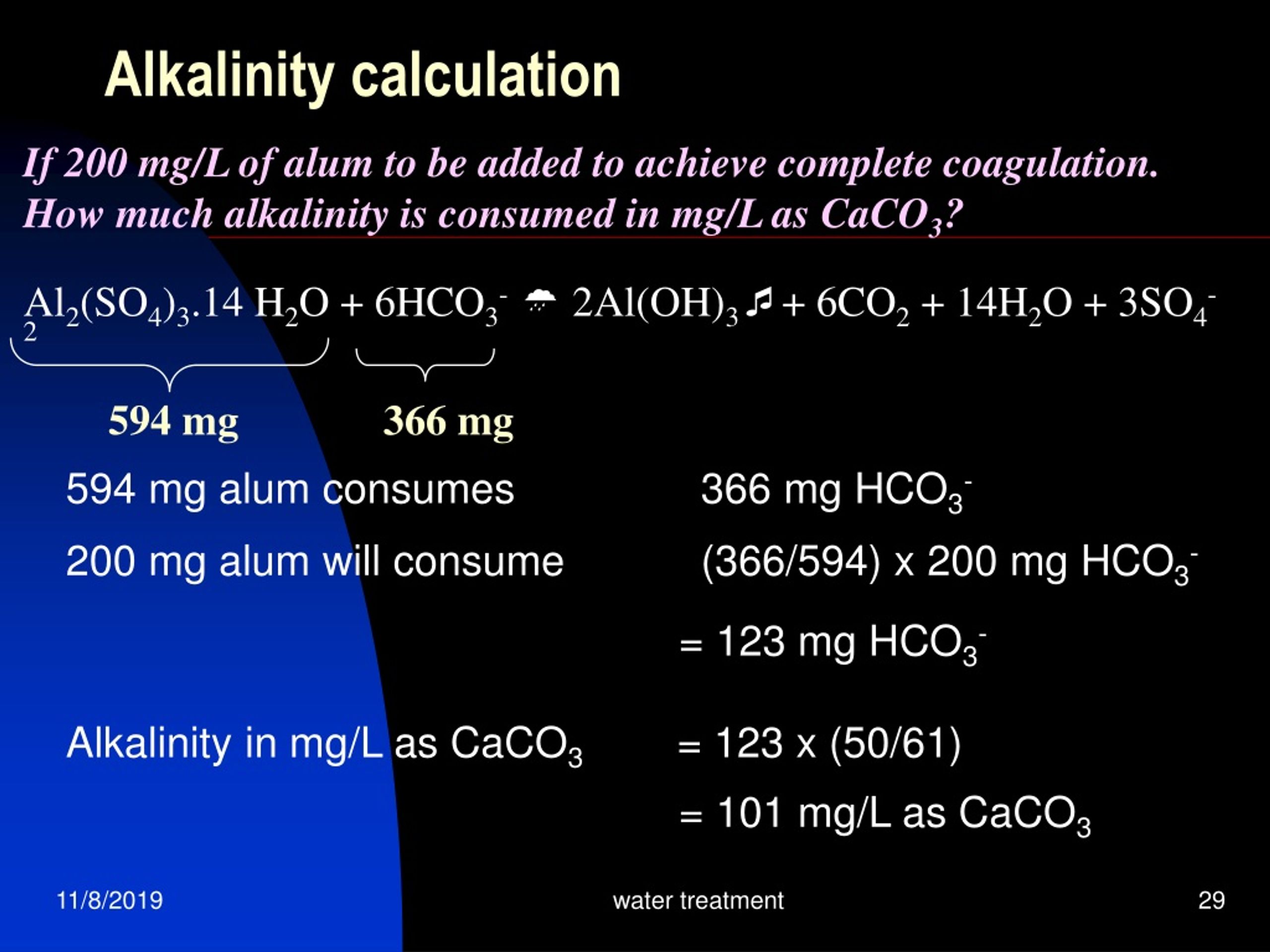
Why alkali is added to the nitrification tank?
In addition, nitrification is pH-sensitive and rates of nitrification will decline significantly at pH values below 6.8. Therefore, it is important to maintain an adequate alkalinity in the aeration tank to provide pH stability and also to provide inorganic carbon for nitrifiers.
What is the role of alkalinity in the wastewater treatment process?
The biological wastewater treatment process also generates hydrogen ions, and so alkalinity is needed to keep the pH of the solution in the required range. If the alkalinity is too low then the extra hydrogen ions are not removed, the pH drops, and the speed of the wastewater treatment slows or even stops.Jan 18, 2018
When an alkaline substance is added to sewage What is the pH?
between 7-8In Wastewater If all alkalinity in the wastewater process is consumed, an alkaline solution such as caustic soda or magnesium hydroxide can be added to maintain the system pH between 7-8 as the denitrifying bacteria generate acid but this adds cost and complexity to the system.
How do you add alkalinity to wastewater?
Common chemicals used to increase alkalinity and pH include: Calcium oxide or calcium hydroxide (as lime slurry) Sodium hydroxide (caustic soda) Sodium carbonate (soda ash) or sodium bicarbonate.
What contributes to alkalinity?
The main sources for natural alkalinity are rocks which contain carbonate, bicarbonate, and hydroxide compounds. Borates, silicates, and phosphates may also contribute to alkalinity.
At what pH range alkalinity is present in water?
Pure water has a pH of 7.0 and is neutral; water measuring under 7.0 is acidic; and that above 7.0 is alkaline or basic.
How does alkalinity affect pH?
Alkalinity tells you the buffering capacity in the basic pH range of the water. You can have a high (or low) pH water with very little buffering capacity, meaning you can easily and quickly change the pH of the water; this also means the water is unlikely to change the pH of soils or potting mixes.Apr 12, 2018
How does pH affect wastewater treatment?
The term “pH” refers to the measurement of hydrogen ion activity in the solution. Determination of pH plays an important role in the wastewater treatment process. Extreme levels, presence of particulate matters, accumulation of toxic chemicals and increasing alkalinity levels are common problems in wastewater.Aug 2, 2016
Which process is used to treat highly acidic and alkaline water?
How soda ash/sodium hydroxide injection works. This treatment method is used if water is acidic (low pH). Soda ash (sodium carbonate) and sodium hydroxide raise the pH of water to near neutral when injected into a water system.Aug 23, 2019
How does titration determine the alkalinity of water?
Alkalinity is determined by titrating a water sample with a strong acid (such as chlorine and sulfuric acid) and expressed by the calcium carbonate content (mg/L) corresponding to the amount of acid consumed until the pH value reaches the prescribed value. Alkalinity measured with the end point of pH4.
How do you control alkalinity in wastewater?
Alkalinity and pH can be dramatically raised and controlled through the use of soda ash (sodium carbonate) where baking soda barely effects the ove...
How can alkalinity be controlled?
In order to control alkalinity, you must control the pH in order to resist changes that can occur. ... pH adjustment alters the pH or Potential Hyd...
How do you treat alkalinity in water?
Common chemicals used to increase alkalinity and pH include: Calcium oxide or calcium hydroxide (as lime slurry) Sodium hydroxide (caustic soda) So...
How does alkalinity affect wastewater?
Alkalinity can be defined as the ability of a water to neutralize acid or to absorb hydrogen ions. It is the sum of all acid neutralizing bases in...
What causes alkalinity in wastewater?
In municipal and industrial wastewater there are many factors which contribute to alkalinity. Factors which contribute to alkalinity include the ty...
Alkalinity and Acid Neutralizing Capacity
Running alkalinity in mobile lab when working on Muddy Creek, eastern Utah, Oct 2015.
Why alkalinity is important
Although you don't often hear about the alkalinity of your favorite lake in the news, alkalinity can be important to the health and welfare of a lake. The ecosystem and organisms that live in the lake evolved in water bodies that didn't change quickly. Before humans came along water bodies were not subjected to chemical spills and acid rain.
What affects alkalinity?
In a surface water body, such as a lake, the alkalinity in the water comes mostly from the rocks and land surrounding the lake. Precipitation falls in the watershed surrounding the lake and most of the water entering the lake comes from runoff over the landscape.
Map of alkalinity in surface waters in the U.S
Here is a map made by the U.S. Environmental Protection Agency (EPA) that shows alkalinity values for surface waters the United States. According to the EPA, this map provides a general illustration of the national patterns of surface-water alkalinity in the conterminous United States.
What are the two types of alkalinity?
Types of Alkalinity: Two types of Alkalinity present in water, P-Alkalinity also called Phenolphthalein Alkalinity because Phenolphthalein indicator used for analysis. M-Alkalinity also called Methyl orange Alkalinity because Methyl orange indicator used for analysis. In alkalinity analysis different ions can be estimated separately by doing ...
What does P mean in alkalinity?
1.Phenolphthalein alkalinity (P) = 0; that means the volume of acid used till the completion of reaction (1) and (2) is 0. This can only happen when both OH– and CO32– ions are not present in water. Alkalinity is present due to HCO3– ion only which can be determined using methyl orange indicator and called methyl orange alkalinity (M).
Why does water absorb H+ ions?
When you add acid in water (adding H + ions) water absorbs H + ions without showing significant change in pH. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water.
How Alkalinity Affects Nitrification
Use alkalinity profiling in wastewater operations to control biological activity and optimize process control The Water Environment Federation’s new Operations Challenge laboratory event will determine alkalinity needs to facilitate nitrification.
NeoWaterFX300, pH and Alkalinity
This ability to maintain proper pH in the wastewater as it undergoes treatment is the reason why alkalinity is so important to the wastewater process.
Coagulation and Flocculation in Water and Wastewater Treatment
Coagulation and flocculation are an essential part of drinking water treatment as well as wastewater treatment. This article provides an overview of the processes and looks at the latest thinking.
5.10 Total Alkalinity
A buret is a long, graduated glass tube with a tapered tip like a pipet and a valve that is opened to allow the reagent to drip out of the tube. The amount of reagent used is calculated by subtracting the original volume in the buret from t he volume left after the endpoint has been reached. Alkalinity is calculated based on the amount used.
pH of Water
pH stand for the “power of hydrogen” and is a logarithmic scale for how acidic or basic water is. Low numbers are acidic, high numbers basic.
Why pH Is Important in Wastewater Treatment
The term “pH” refers to the measurement of hydrogen ion activity in the solution.
White Paper: The Role Of Alkalinity In Aerobic Wastewater Treatment Plants: Magnesium Hydroxide vs. Caustic Soda
This paper will discuss the role that alkalis play in wastewater treatment.
Why do teeth pit?
Tooth discoloration and/or pitting is caused by excess fluoride exposures during the formative period prior to eruption of the teeth in children. The secondary standard of 2.0 mg/L is intended as a guideline for an upper boundary level in areas which have high levels of naturally occurring fluoride.
What are the problems with water?
These problems can be grouped into three categories: 1 Aesthetic effects — undesirable tastes or odors; 2 Cosmetic effects — effects which do not damage the body but are still undesirable 3 Technical effects — damage to water equipment or reduced effectiveness of treatment for other contaminants
What is the EPA drinking water regulation?
EPA has established National Primary Drinking Water Regulations National Primary Drinking Water Regulations Legally enforceable standards that apply to public water systems. These standards protect drinking water quality by limiting the levels of specific contaminants that can adversely affect public health and which are known or anticipated ...
Why do disinfectants have color?
Color may be indicative of dissolved organic material, inadequate treatment, high disinfectant demand, and the potential for the production of excess amounts of disinfectant by-products. Inorganic contaminants such as metals are also common causes of color.
What is the best treatment for odors?
Coagulation (or flocculation) and filtration removes metals like iron, manganese and zinc. Aeration removes odors, iron, and manganese. Granular activated carbon will remove most of the contaminants which cause odors, color, and foaming.
What are the standards related to color?
Standards related to color: Aluminum, Color, Copper, Iron, Manganese, Total Dissolved Solids. Foaming is usually caused by detergents and similar substances when water has been agitated or aerated as in many faucets. An off-taste described as oily, fishy, or perfume-like is commonly associated with foaming.
What are the effects of corrosive water?
Other effects of corrosive water, such as the corrosion of iron and copper, may stain household fixtures and impart objectionable metallic taste and red or blue-green color to the water supply. Corrosion of distribution system pipes can reduce water flow.
Where to apply sodium bicarbonate in anaerobic system?
With anaerobic systems, it is recommended that product be applied in the wet well or scum pit on the sludge line leading to the digester. Sodium bicarbonate should be fed in a manner that promotes maximum distribution throughout the digester.
Why is buffering important in secondary treatment plants?
It provides a reserve buffering ability to help prevent upsets in the operating systems of secondary treatment plants that depend on microorganisms to digest organic wastes and control odor . Acids produced in the waste breakdown process, along with other conditions prevalent in treatment plants tend to lower pH.
What happens if there is not enough alkalinity to support nitrification?
If there is not enough influent alkalinity to support nitrification, you may find an increase in nitrite exiting the biological treatment process. This might be at certain times of the day, or on certain days of the week, depending on your flows and influent characteristics.
How much nitrate nitrogen does CaCO3 lose?
suspicion will be confirmed if you see that the alkalinity is very low (<40 mg/l as CaCO3), the ammonia/ammonium levels have increased, nitrite is present, and nitrate nitrogen has decreased. Just 1 to 2 mg/l of nitrite is all it takes to lose 5 to 10 mg/l of chlorine residual.
What do nitrifiers do in wastewater?
Inside a wastewater treatment plant, autotrophic bacteria (nitrifiers) consume alkalinity and oxidize ammonia to gain energy for reproduction . With enough dissolved oxygen (DO), adequate temperatures, the right detention time and several other items, nitrifiers convert ammonia and ammonium to nitrite (NO2-), and then nitrate (NO3-).
Why do you see a spike in nitrate?
This happens because the nitrite was oxidized by the chlorine to nitrate and used up in the reaction . You need to measure nitrite and nitrate just before the chlorine injection point.
Why is chlorine not maintained?
There are several reasons why a treatment plant might not be able to maintain chlorine residual: excessive chlorine demand, improper effluent pH, disinfectant feed equipment malfunction, and interferences with the residual chlorine testing reagents.
How much bicarbonate alkalinity is needed for nitrate reduction?
During the biological reduction of nitrate, a small amount (about 3.5 mg/l) of bicarbonate alkalinity is re-established, helping to increase effluent alkalinity, and the interference of nitrite is reduced as well. So there you have it.
How much alkalinity is needed for nitrification?
If your influent ammonia is around 30 mg/l, you need 213 mg/l of influent alkalinity to complete nitrification. Remember that this is a minimum — you still need some for acid buffering in downstream processes, like disinfection.
What is the difference between Neo Waterfx 300 and Neo Waterfx 300?
In simpler terms, the average iron/aluminum solution is 75-100 times more acidic than Neo WaterFX 300 (formerly RE300). The second advantage Neo WaterFX 300 (formerly RE300) has is the dose volume. Neo WaterFX 300 (formerly RE300) typically replaces iron/aluminum with a dose volume 25% or less. These two mechanisms combine to result in a reduction of acid addition of 300-500 times. If each acidic H+ molecule contributes to alkalinity depletion, switching from ferric chloride to NeoWaterFX 300 could reduce chemical alkalinity consumption by several hundred times leaving more alkalinity for denitrification.
Why is alkalinity important in wastewater treatment?
This ability to maintain the proper pH in the wastewater as it undergoes treatment is the reason why alkalinity is so important to the wastewater process. If all alkalinity in the wastewater process is consumed , an alkaline solution such as caustic soda or magnesium hydroxide can be added to maintain the system pH between 7-8 as ...
What is Neo Waterfx 300?
Neo WaterFX 300 (formerly RE300) is a rare earth-based coagulant for use in water treatment. It is mainly composed of the ionic forms of Cerium (Ce) but can contain other rare earths such as Lanthanum (La), Neodymium (Nd), and Praseodymium (Pr) among others. Neo WaterFX 300 (formerly RE300) is stable in a solution with a pH between 3 and 4. One of the many benefits of NeoWaterFX 300 it is significantly less acidic than other metal salts used in water treatment. This document will focus on reasons why a less acidic chemical can be a benefit in water treatment. The following definitions are given to help clear up some confusion on the subjects of acid/base chemistry and alkalinity.
What pH is needed for wastewater treatment?
The bacteria and other organisms which play an active role in wastewater treatment are most effective at a neutral to slightly alkaline pH of 7 to 8. To maintain these optimal pH conditions for biological activity there must be sufficient alkalinity present in the wastewater to neutralize acids generated by the active biomass during waste treatment especially nitrification. This ability to maintain the proper pH in the wastewater as it undergoes treatment is the reason why alkalinity is so important to the wastewater process. If all alkalinity in the wastewater process is consumed, an alkaline solution such as caustic soda or magnesium hydroxide can be added to maintain the system pH between 7-8 as the denitrifying bacteria generate acid but this adds cost and complexity to the system.
Why does acidic water react with alkalinity?
The acid molecules react with the alkalinity which results in the acid molecules being neutralized, therefor when adding acid to a solution with alkalinity, the pH stays constant until the alkalinity is consumed . This is the reason adding acidic water treatment chemicals consumes alkalinity.
What is the difference between acid and base?
A pH of 1 is the lowest number on the scale and therefor the most acidic measurement on the pH scale. Base – A base is anything that will release a hydronium ion (OH-) in solution, or anything that will consume an acid H+.
What is the pH range of a solution?
It ranges from 1 to 14 with 7 considered neutral. The pH scale is logarithmic which means that every integer change results in a 10x higher acid or base concentration. Example; pH 6 is 10 times more acidic than pH 7 and pH 4 is 1,000 times more acidic than 7. Alkalinity – Alkalinity is the ability of a solution to resist pH changes ...
What is the function of alkalinity in wastewater?
Alkalinity is often used as an indicator of biological activity . In wastewater operations, there are three forms of oxygen available to bacteria: dissolved oxygen (O 2 ), nitrate ions (NO 3– ), and sulfate ions (SO 42- ). Aerobic metabolisms use dissolved oxygen to convert food to energy. Certain classes of aerobic bacteria, called nitrifiers, use ammonia (NH 3) for food instead of carbon-based organic compounds. This type of aerobic metabolism, which uses dissolved oxygen to convert ammonia to nitrate, is referred to as “nitrification.” Nitrifiers are the dominant bacteria when organic food supplies have been consumed.
What is the Water Environment Federation's new Operations Challenge Laboratory Event?
The Water Environment Federation’s new Operations Challenge laboratory event will determine alkalinity needs to facilitate nitrification. Operators will evaluate alkalinity and ammonia by analyzing a series of samples similar to those observed in water resource recovery facilities.
What happens to lignin during acidic sulfite pulping?
2. Delignification during acid treatment. During acidic sulfite pulping, the main reaction that happens to lignin comes from hydrion and hydrated sulfur dioxide. The major part of sulfonation is C α, C γ occasionally, which increases the solubility of lignin.
What is alkaline treatment?
The alkaline treatment or mercerization is a chemical treatment in which the natural fibers are immersed in a known concentration of aqueous sodium hydroxide (NaOH) for a given temperature and a period of time. The alkaline treatment modifies the surface of fibers by removing a certain rate of lignin, hemicellulose, wax, and oils covering the external surface of natural fibers [26]. For example, in the fibers of hemp, the alkaline treatment completely eliminates pectin without a residue, but the remaining lignin depends on the concentration of NaOH [27]. The partially removed lignin wax and hemicellulose enhance the matrix–fiber interface and ensure good adhesion between the matrix and natural fibers. If the treatment parameters are not optimized, the mercerization can cause fiber defibrillation, pore formation, and fiber embrittlement [28,29].
What is the characteristic of alkali delignification?
In the alkaline cooking process, a characteristic of delignification is that lignin macromolecules must be broken into small molecules, so that they can be dissolved in the solution. Therefore, the alkali delignification process is the cleavage reaction of various bonds between the units of lignin macromolecules.
What is bleaching used for?
Bleaching is applied after alkaline treatment to remove all elements containing chromophore groups and phenolic compounds as lignin and hemicellulose. The aim is to isolate cellulose from the natural fiber by using chemical agents such as hydrochloric acid, sodium chlorite, sodium hydroxide, and chlorine dioxide (H 2 O 2 ). Hydrochlorous acid (HClO 2 ), which is generated by the dissociation of NaCiO 2 in a distilled water medium, is largely used as a bleaching reagent [39]. The mechanisms of bleaching reaction are noted by these equations.
What is the main product of lignin chromophoric groups?
During alkaline and acidic sulfite digesting, the main product of lignin chromophoric groups is diarylbenzenes. Catechol is obtained due to the removal of the methoxy group, and it is then oxidized into diquinone or forms dark compounds with metal ions. Finally, the stilbene structure is formed. 3.
What is mercerization used for?
Mercerization is one of the chemical treatments of natural fibers most commonly used to reinforce thermoplastics and thermosets. The important modification resulting from alkaline treatment is the disruption of hydrogen bonding in the network structure, thereby increasing surface roughness.
What is the chemical used to bleach plant fibers?
Alkaline treatment or mercerization (ASTM D1695) is defined as the process of subjecting a vegetable fiber to the action of a fairly concentrated aqueous solution of a strong base to produce great swelling with resultant changes in the fine structure, dimension, morphology, and mechanical properties. Sodium hydroxide (NaOH) is the most commonly used chemical for bleaching and/or cleaning the surface of plant fibers and it also changes the fine structure of native cellulose I to cellulose III, with depolymerization and the production of short length crystallites. The basic fiber properties such as strength and elongation at break can be changed by a suitable choice of mercerization parameters.