What is the cost of the newest hepatitis C drug?
2010 wholesale acquisition costs: 41,803: The total treatment cost input was a function of the regimen used for each genotype in the given year weighted by the prevalence of each genotype. Regimen list prices were identified in RED BOOK. 21 2012 wholesale acquisition costs: 97,196 2014 wholesale acquisition costs: 106,360 2016 wholesale acquisition costs: 68,753
Who pays for HCV treatment?
Dec 13, 2019 · The cost-effectiveness, health benefits, and financial costs of new antiviral treatments for hepatitis C virus. Clin Infect Dis . 2015;61(2):157-168. doi: 10.1093/cid/civ220. 23.
Can I get help paying for hepatitis C drugs?
Jun 01, 2018 · Hepatitis C virus (HCV) is transmitted by exposure to blood or other bodily fluids that contain HCV. Approximately 3.5 million Americans have chronic hepatitis C. About 19,000 of these people die ...
Does insurance cover hepatitis C treatments?
Jan 19, 2016 · Screening costs included the cost of HCV antibody, HCV RNA, HCV genotype tests, and FibroSure test. Treatment costs included the wholesale acquisition cost of sofosbuvir ($7000 per week), ledipasvir ($1125 per week), and ribavirin ($309 per week) , and we considered drug discounts in sensitivity analysis . HCV disease management costs included the cost …

How to pay for HCV?
If you’re concerned about paying for HCV medications, remember that you aren’t alone as you seek treatment. There are people and organizations that can help you, including the following: 1 Your doctor. They can help you by ordering and documenting the tests you’ll need so you can qualify to get your medications, especially if you’re working with a liver or infection specialist. 2 Most drug manufacturers. There are patient assistance programs that offer free or reduced-cost medications for people who meet their criteria. 3 Patient advocacy groups. These groups provide assistance with all aspects of HCV treatment. For instance, if your insurer denies treatment, you can appeal the decision with help from one of these groups. Your doctor can also help in this situation.
How many people die from hepatitis C each year?
Americans have chronic hepatitis C. About 19,000 of these people die each year from cirrhosis or liver cancer. Fortunately, recent advancements in the fight against this virus have changed the outlook for people with HCV. New drugs have transformed the disease from one that can, at best, be controlled to one that can be cured for most people who ...
What is the liver infection?
Hepatitis C is a viral infection that attacks the liver. Infection with hepatitis C can lead to serious liver disease, including cirrhosis and cancer. Hepatitis C virus (HCV) is transmitted by exposure to blood or other bodily fluids that contain HCV.
What is a direct acting antiviral?
of people who take them, depending on the type of HCV infection and treatment exposure. These new drugs are called direct-acting antivirals (DAAs). The U.S. Food and Drug Administration (FDA) approved the first of these medications for HCV treatment in 2011. Several more medications have been approved since that time.
Is generic medicine cheaper than brand name?
It also means there are no generic versions of these drugs yet. Generics are typically much cheaper than brand- name versions. The FDA determines how long this period of exclusivity will last. During this time, the pharmaceutical companies have a lot of freedom in establishing prices.
What are the criteria for liver disease?
These criteria may be based on: the severity of liver disease. whether the person avoids alcohol and drug use. whether the drug’s prescribed by a doctor who specializes in liver diseases. the life expectancy of the person seeking treatment. whether less expensive treatments could be used first.
Can hepatitis C be treated with drugs?
Today there are several drug options available that can cure hepatitis C infection — that’s the great news. What’s less great is the high cost of these drugs. However, there are many options you can explore to find help paying for these medications.
What is cost effectiveness analysis?
Cost-effectiveness analysis (CEA) compares the relative costs and outcomes of 2 or more interventions. CEA explicitly recognizes budget limitations for healthcare spending and seeks to maximize public health benefits within those budgetary constraints. The core question that CEA addresses is whether to invest limited healthcare dollars in a new treatment/therapy or use that money to invest in another healthcare intervention that would provide better outcomes for the same monetary investment. The focus of CEA is, therefore, not simply cost or saving money but health benefits. It assumes that all available resources will be spent and provides a framework for prioritizing among available treatment options by formally assessing the comparative costs and health benefits accrued from a new treatment relative to current treatment.
What is the time horizon for CEA?
From a societal perspective, CEA uses a lifetime time horizon, meaning it considers lifetime costs and benefits, including those that occur in the distant future. Business budget planning, however, typically assumes a 1-year to 5-year perspective.
What does private insurance do?
Private insurance companies often have separate pharmacy and medical budgets, and use PBMs or directly negotiate drug pricing with pharmaceutical companies. Insurance companies determine formulary placement, which impacts the choice of regimens and out-of-pocket expenses for patients.
Is life expectancy a measure of benefit?
Life expectancy is a valuable measure of benefit but considering only mortality benefits fails to recognize the value of treatments that improve quality of life. The quality-adjusted life-year (QALY) provides a measure that integrates both longevity and quality of life and is the preferred outcome for CEA.
Is an intervention cost effective?
An intervention that is cost-effective is not necessarily affordable. Affordability refers to whether a payer has sufficient resources in its annual budget to pay for a new therapy for all who might need or want it within that year . Several characteristics of CEA limit its ability to speak to the budgetary impact of interventions being implemented in the real world.
Is HCV cost effective?
There is no formula that provides a good means of integrating the concerns of value and affordability. When new HCV therapies are deemed cost-effective, it indicates that these therapies provide good benefit for the resources invested and providing such therapy to more people would be a good long-term investment.
Is routine HCV testing cost effective?
Generally, routine HC V testing is cost-effective because the incidence and prevalence of HCV remain high in people who inject drugs with a notable rising prevalence in young adults who may not readily report their stigmatized risk behaviors.
How much does it cost to cure hepatitis C?
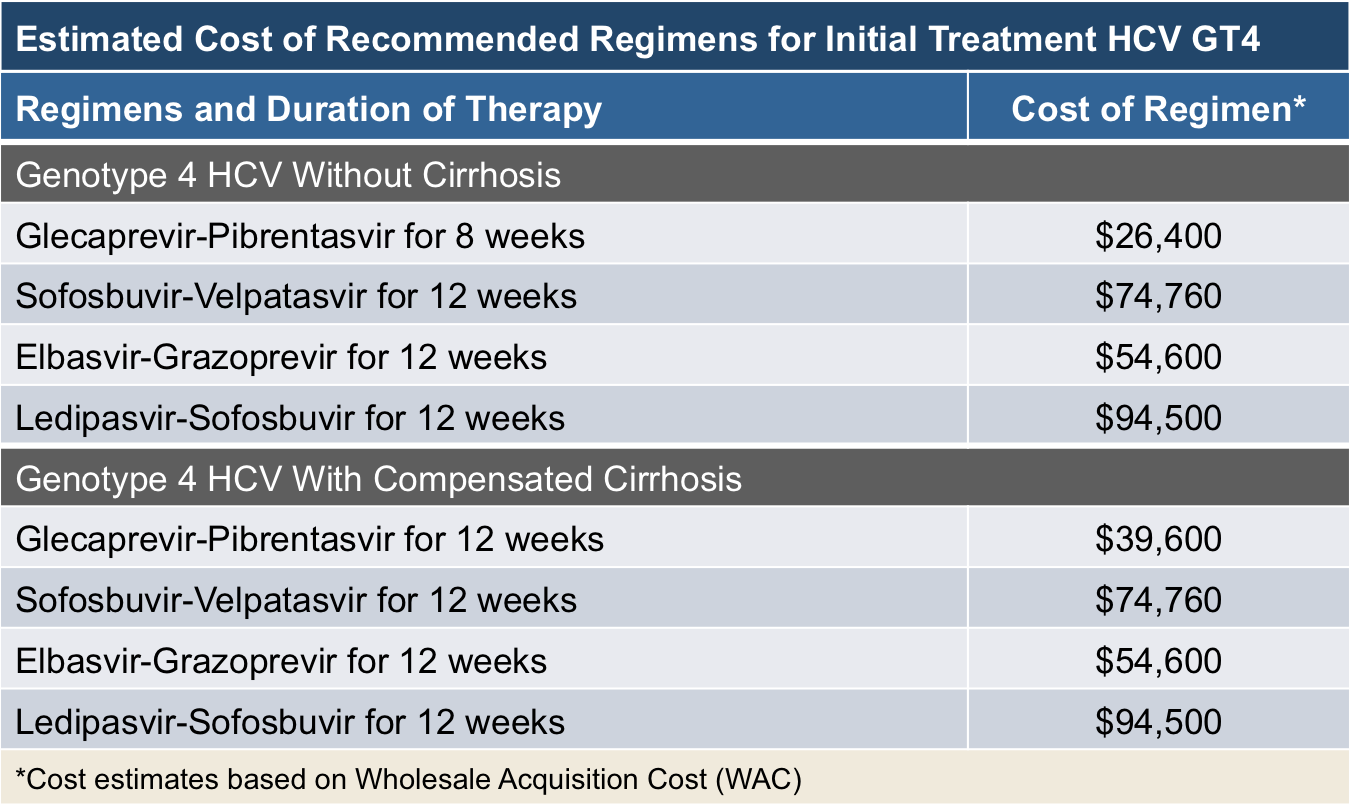
According to the Pharmacy Times, the cost of treatment can be as low as $54,600 for the 12-week course and the entry to the market of new, cheaper drugs is likely to continue to bring the cost of hepatitis C treatments down.
How much does Sovaldi cost?
Sofosbuvir (Sovaldi): This medication costs $1,000 per 400 mg pill. The total cost for a 12-week course is around $84,000, and doctors will typically prescribe it with other medicines, such as simeprevir. Ombitasvir-paritaprevir-ritonavir and dasabuvir (Viekira Pak): The cost for this medication is $83,319 for a 12-week treatment course.
Is hepatitis C treatment successful?
Recent innovations in hepatitis C treatment mean that treatment is usually successful. However, for some people, the costs of these medications can be prohibitive. While prices may decrease in the future, there are currently no guarantees.
Is hepatitis C a direct-acting drug?
In 2014, the United States Food and Drug Administration (FDA) approved drug treatment s called direct-acting antiviral medications that were available in oral form only to treat hepatitis C, according to the journal Open Forum Infectious Diseases . Several medications to treat hepatitis C have met FDA approval since then.