How much does hepatitis C treatment cost?
RESULTS: No HCV treatment resulted in a gain of 11.54 QALYs over a 20-year time horizon at a cost of $42,938. Costs for treated groups were $69,075, $123,267, $125,431, $86,782, and $56,470 for the 2010, 2012, 2014, 2016, and 2018 scenarios, respectively. QALYs gained for treated groups were
Who pays for HCV treatment?
2010 wholesale acquisition costs: 41,803: The total treatment cost input was a function of the regimen used for each genotype in the given year weighted by the prevalence of each genotype. Regimen list prices were identified in RED BOOK. 21 2012 wholesale acquisition costs: 97,196 2014 wholesale acquisition costs: 106,360 2016 wholesale acquisition costs: 68,753
What are the new drugs available to treat hepatitis C?
Jun 01, 2018 · Hepatitis C virus (HCV) is transmitted by exposure to blood or other bodily fluids that contain HCV. Approximately 3.5 million Americans have chronic hepatitis C. About 19,000 of these people die ...
How has hepatitis C treatment evolved?
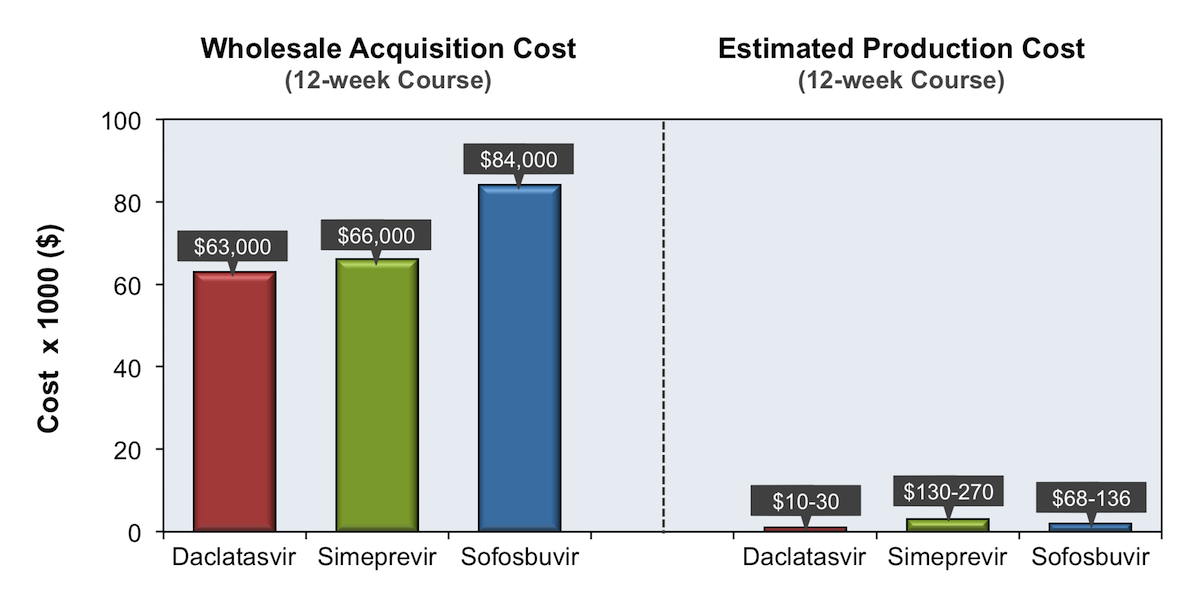
The wholesale acquisition cost for the newer DAAs ranges from $417 to $1,125 per day. The actual price paid for the medication may be significantly lower …

How much does Sovaldi cost in America?
Official Answer. The wholesale cost of Sovaldi is $1000 per 400mg tablet. A 12-week treatment course of Sovaldi costs around $84,000 and a 24-week course, $168,00.Apr 23, 2020
How much does maverick for hep C cost?
Abbvie has priced Mavyret at $13,200 per month, or $26,400 per treatment course, before discounts. Although this is still expensive, Macyret is priced significantly lower than other hepatitis C treatments.Aug 23, 2017
How much do direct acting antivirals cost?
Conclusions: Within the next 15 years, large-scale manufacture of 2 or 3 drug combinations of HCV DAAs is feasible, with minimum target prices of $100-$250 per 12-week treatment course. These low prices could make widespread access to HCV treatment in low- and middle-income countries a realistic goal.
What company makes MAVYRET?
AbbVie Receives U.S. FDA Approval of MAVYRET™ (glecaprevir/pibrentasvir) for the Treatment of Chronic Hepatitis C in All Major Genotypes (GT 1-6) in as Short as 8 Weeks.Aug 3, 2017
Is MAVYRET a generic?
No. There is currently no therapeutically equivalent version of Mavyret available in the United States. Note: Fraudulent online pharmacies may attempt to sell an illegal generic version of Mavyret. These medications may be counterfeit and potentially unsafe.Mar 10, 2022
How much does Maviret cost?
GLECAPREVIR + PIBRENTASVIRCode & PrescriberMedicinal Product Pack (Name, form & strength and pack size)General Patient Charge11354NGLECAPREVIR + PIBRENTASVIR glecaprevir 100 mg + pibrentasvir 40 mg tablet, 84 (PI, CMI)$42.50Available brandsMaviret
How much is hep C treatment in India?
The generic version of these drugs are available in cities such as Bengaluru Hyderabad and Chennai at the cost of Rs70000 or around $1000 USD for the entire treatment regimen.
How much does hep C treatment cost UK?
A 12-week course of treatment with elbasvir-grazoprevir usually costs £36,500 per patient, but the NHS will pay less than this as the company has offered a confidential discount. Taken once daily, the tablet could treat around 4,000 patients in the first year, alongside other options already available for hepatitis C.
Is Hep C curable 2020?
Hepatitis C (hep C) infection used to be a lifelong condition for most people. Up to 50 percent of people may clear the hepatitis C virus (HCV) from their body without treatment. For everyone else, the infection becomes chronic. With advances in hep C treatment, most people can now be cured of HCV.
Is MAVYRET better than Harvoni?
Mavyret is reported to have some advantages over Harvoni including the number of HCV genotypes it covers, the length of treatment required, and the cost of a course of treatment.Aug 25, 2021
What is Maverick for hep C?
MAVYRET is a prescription medicine used to treat adults and children 12 years of age and older or weighing at least 99 pounds (45 kilograms) with chronic (lasting a long time) hepatitis C virus (hep C): Genotypes (GT) 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis.
What is the new medicine for hep C?
The new hepatitis C treatments are sofosbuvir with ledipasvir (Harvoni); sofosbuvir (Sovaldi); daclatasvir (Daklinza); and ribavirin (Ibavyr). These new treatments are now available on the Pharmaceuticals Benefits Scheme.Mar 1, 2016
What is the primary analysis for this methodology study focused on?
The primary analysis for this methodology study focused on the changing costs and effectiveness estimates at each time point to estimate incremental cost-effectiveness ratios. A scenario analysis was conducted using only the WAC for each drug referenced in RED BOOK to describe the effect of using list versus net price in the CEA. 21
Is HCV treatment effective?
Treatment effectiveness for HCV has increased steadily, while treatment costs increased substantially from 2010-2014 before decreasing to its lowest point in 2018. The dynamic nature of CEAs in a disease state with rapid pharmaceutical innovation may cause some concern for decision makers who rely on a single analysis over time. Model transparency along with resources to update or revise model assumptions would enable organizations to provide more up-to-date results to inform formulary decisions.
How to pay for HCV?
If you’re concerned about paying for HCV medications, remember that you aren’t alone as you seek treatment. There are people and organizations that can help you, including the following: 1 Your doctor. They can help you by ordering and documenting the tests you’ll need so you can qualify to get your medications, especially if you’re working with a liver or infection specialist. 2 Most drug manufacturers. There are patient assistance programs that offer free or reduced-cost medications for people who meet their criteria. 3 Patient advocacy groups. These groups provide assistance with all aspects of HCV treatment. For instance, if your insurer denies treatment, you can appeal the decision with help from one of these groups. Your doctor can also help in this situation.
How many people die from hepatitis C each year?
Americans have chronic hepatitis C. About 19,000 of these people die each year from cirrhosis or liver cancer. Fortunately, recent advancements in the fight against this virus have changed the outlook for people with HCV. New drugs have transformed the disease from one that can, at best, be controlled to one that can be cured for most people who ...
What is the liver infection?
Hepatitis C is a viral infection that attacks the liver. Infection with hepatitis C can lead to serious liver disease, including cirrhosis and cancer. Hepatitis C virus (HCV) is transmitted by exposure to blood or other bodily fluids that contain HCV.
What is a direct acting antiviral?
of people who take them, depending on the type of HCV infection and treatment exposure. These new drugs are called direct-acting antivirals (DAAs). The U.S. Food and Drug Administration (FDA) approved the first of these medications for HCV treatment in 2011. Several more medications have been approved since that time.
Is generic medicine cheaper than brand name?
It also means there are no generic versions of these drugs yet. Generics are typically much cheaper than brand- name versions. The FDA determines how long this period of exclusivity will last. During this time, the pharmaceutical companies have a lot of freedom in establishing prices.
What are the criteria for liver disease?
These criteria may be based on: the severity of liver disease. whether the person avoids alcohol and drug use. whether the drug’s prescribed by a doctor who specializes in liver diseases. the life expectancy of the person seeking treatment. whether less expensive treatments could be used first.
Can hepatitis C be treated with drugs?
Today there are several drug options available that can cure hepatitis C infection — that’s the great news. What’s less great is the high cost of these drugs. However, there are many options you can explore to find help paying for these medications.
What is cost effectiveness analysis?
Cost-effectiveness analysis (CEA) compares the relative costs and outcomes of 2 or more interventions. CEA explicitly recognizes budget limitations for healthcare spending and seeks to maximize public health benefits within those budgetary constraints. The core question that CEA addresses is whether to invest limited healthcare dollars in a new treatment/therapy or use that money to invest in another healthcare intervention that would provide better outcomes for the same monetary investment. The focus of CEA is, therefore, not simply cost or saving money but health benefits. It assumes that all available resources will be spent and provides a framework for prioritizing among available treatment options by formally assessing the comparative costs and health benefits accrued from a new treatment relative to current treatment.
What is the time horizon for CEA?
From a societal perspective, CEA uses a lifetime time horizon, meaning it considers lifetime costs and benefits, including those that occur in the distant future. Business budget planning, however, typically assumes a 1-year to 5-year perspective.
What does private insurance do?
Private insurance companies often have separate pharmacy and medical budgets, and use PBMs or directly negotiate drug pricing with pharmaceutical companies. Insurance companies determine formulary placement, which impacts the choice of regimens and out-of-pocket expenses for patients.
Is life expectancy a measure of benefit?
Life expectancy is a valuable measure of benefit but considering only mortality benefits fails to recognize the value of treatments that improve quality of life. The quality-adjusted life-year (QALY) provides a measure that integrates both longevity and quality of life and is the preferred outcome for CEA.
Is an intervention cost effective?
An intervention that is cost-effective is not necessarily affordable. Affordability refers to whether a payer has sufficient resources in its annual budget to pay for a new therapy for all who might need or want it within that year . Several characteristics of CEA limit its ability to speak to the budgetary impact of interventions being implemented in the real world.
Is HCV cost effective?
There is no formula that provides a good means of integrating the concerns of value and affordability. When new HCV therapies are deemed cost-effective, it indicates that these therapies provide good benefit for the resources invested and providing such therapy to more people would be a good long-term investment.
Is routine HCV testing cost effective?
Generally, routine HC V testing is cost-effective because the incidence and prevalence of HCV remain high in people who inject drugs with a notable rising prevalence in young adults who may not readily report their stigmatized risk behaviors.
What is the role of Daclatasvir in hepatitis C?
Daclatasvir was discovered as a first-in-class inhibitor of the non-structural viral protein 5A (NS5A), a phosphoprotein that plays an important role in hepatitis C replication. The exact mechanism by which daclatasvir inhibits the NS5A replication complex is unclear, but it is believed that daclatasvir inhibits viral RNA replication and virion assembly. It may also inhibit phosphorylation of the NS4A, and therefore the formation and activation of the HCV replication complex. Based on in vitro data, daclatasvir has shown activity against HCV genotypes 1 through 6, with EC50 values ranging from picomolar to low nanomolar against wild type HCV.
How much ribavirin should I take for cirrhosis?
When ribavirin is used in patients with HCV genotype 1 or 3 and either Child-Pugh B or C cirrhosis or post-transplantation patients, the recommended initial dose of ribavirin is 600 mg once daily, increasing up to 1000 mg daily as tolerated. In this scenario, the ribavirin starting dose and on-treatment dose can be decreased based on hemoglobin ...
How much Daclatasvir should I take daily?
The recommended standard dose of daclatasvir is 60 mg orally once daily, with or without food. The recommended dose of sofosbuvir, when used with daclatasvir is 400 mg once daily, with or without food. Dose Modifications of Daclatasvir with CYP3 Inhibitors and Inducers.
Can Daclatasvir be used with Sofosbuvir?
Daclatasvir is indicated for use, with sofosbuvir, with or without ribavirin for the treatment of patients with chronic HCV genotype 1 or 3. The use of ribavirin depends on the patient population treated as outlined below. For patients with HCV/HIV-1 coinfection, the dosage and duration are the same as listed below. Genotype 1.
Is daclatasvir contraindicated?
With Strong CYP3A Inducers: Daclatasvir is contraindicated. Patients with Renal Impairment: The dose of daclatasvir does not need to be adjusted in patients with any degree of renal impairment. In addition, because daclatasvir is highly protein bound, it is unlikely to be removed by dialysis.
Is Daclatasvir a genotype?
Daclatasvir plus sofosbuvir, with or without ribavirin, is an all-oral option for the treatment of genotype 1 or 3 chronic HCV across a variety of patient populations. Based on the results of the phase 3 ALLY trials, daclatasvir and sofosbuvir is an effective, albeit very expensive option for patients with genotype 1 or 3 HCV, including those with cirrhosis, HIV coinfection, or post-liver transplantation. The use of daclatasvir with sofosbuvir has provided an important ribavirin-free oral option for genotype 3 patients, but the 12 week dual therapy has limited efficacy in cirrhotic genotype 3 patients. Cost, lack of coformulation, and the recommendation of baseline NS5A testing in genotype 1a cirrhotic patients make daclatasvir plus sofosbuvir a less compelling option in this subset of patients.

Drug Cost and Reimbursement
- Many organizations are involved with hepatitis C drug distribution and each can impact costs as well as decisions about which regimens are reimbursed (US GAO, 2015); (US CBO, 2015). The roles these organizations have in determining the actual price paid for drugs and who has access to treatment include the following: 1. Pharmaceutical companies determine the wholesale acqui…
Cost-Effectiveness
- Cost-effectiveness analysis (CEA) compares the relative costs and outcomes of 2 or more interventions. CEA explicitly recognizes budget limitations for healthcare spending and seeks to maximize public health benefits within those budgetary constraints. The core question that CEA addresses is whether to invest limited healthcare dollars in a new treatment/therapy or use that …
Affordability
- An intervention that is cost-effective is not necessarily affordable. Affordability refers to whether a payer has sufficient resources in its annual budget to pay for a new therapy for all who might need or want it within that year. Several characteristics of CEA limit its ability to speak to the budgetary impact of interventions being implemented in the real world. 1. Perspective on cost CEA seeks t…
Cost vs Affordability For HCV Treatment
- Despite a growing body of evidence that HCV treatment is cost-effective and may even be cost saving over the long term in some cases, many US payers—especially those offering Medicaid insurance products—continue to limit access to HCV treatment. Access has improved as cost has decreased but limitations remain. Proposed reductions in healthcare spending for Medicaid wou…
Cost-Effectiveness of Screening For HCV
- Several cost-effectiveness studies demonstrate that routine, one-time testing for HCV among all adults in the US would likely identify a substantial number of cases of HCV that are currently being missed, and that doing so would be cost-effective. One study employed simulation modeling to compare several versions of routine guidance, including routine testing for adults over the ages …
Conclusions
- Many studies have demonstrated the economic value of HCV screening (Chaillon, 2019); (Eckman, 2019); (Tasillo, 2019); (Assoumou, 2018); (Barocas, 2018); (Schackman, 2018); (Schechter-Perkins, 2018); (Lyons, 2016); (Hsieh, 2016); (Schackman, 2015) and treatment (Goel, 2018); (Chhatwal, 2017); (He, 2017); (Chahal, 2016); (Chhatwal, 2015); (Chidi, 2016); (Martin, 201…