
The glycolytic inhibitors are particularly effective against cancer cells with mitochondrial defects or under hypoxic conditions, which are frequently associated with cellular resistance to conventional anticancer drugs and radiation therapy.
Could glycolysis be a potential target for cancer treatment?
PET imaging, combined with computed tomography (CT), plays an indispensable role in modern diagnostic oncology. But it is the notion that tumor glycolysis could be used as a potential target for therapy that remain the most intriguing.
What stimulates aerobic glycolysis in cancer cells?
Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64(11):3892–3899. doi: 10.1158/0008-5472.CAN-03-2904.
What is the best approach to target cancer-associated glycans?
We mentioned a few of the diverse and interesting approaches targeting cancer-associated glycans, such as the use of carbohydrate analogs and the development of glycan-specific CAR-T and we focused on HTS for the discovery of small molecules with potential clinical application.
Does inhibition of glycolysis prevent ATP generation in cancer cells?
This metabolic feature has led to the hypothesis that inhibition of glycolysis may severely abolish ATP generation in cancer cells and thus may preferentially kill the malignant cells ( Munoz-Pinedo et al., 2003; Izyumov et al., 2004; Xu et al., 2005b ).
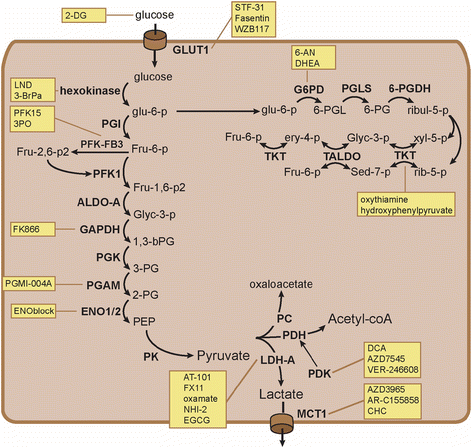
Do cancer cells use glycolysis?
Cancer cells more readily use glycolysis, an inefficient metabolic pathway for energy metabolism, even when sufficient oxygen is available. This reliance on aerobic glycolysis is called the Warburg effect, and promotes tumorigenesis and malignancy progression.
Why does cancer use glycolysis?
Most cancer cells rely on glycolysis to generate ATP, even when oxygen is available. However, merely inhibiting the glycolysis is insufficient for the eradication of cancer cells. One main reason for this is that cancer cells have the potential to adapt their metabolism to their environmental conditions.
What happens to glycolysis in cancer?
Cancer cells exhibit aerobic glycolysis. This means that cancer cells derive most of their energy from glycolysis that is glucose is converted to lactate for energy followed by lactate fermentation, even when oxygen is available. This is termed the Warburg effect.
What are the inhibitors of glycolysis?
Glycolysis Inhibitors2-Deoxy-D-Glucose.3-Bromopyruvic acid.6-Aminonicotinamide.Lonidamine.Oxythiamine Chloride Hydrochloride.Shikonin.
Why do cancer cells use glucose?
All cells need glucose as a source of energy. Normal cells use tiny internal “powerhouses” called mitochondria to convert glucose into units of chemical energy. However, to meet their higher demand for energy, cancer cells have a faster process for metabolizing glucose that does not involve mitochondria.
Do cancer cells rely on glucose?
One of the hallmarks of cancer cell development is the increased dependence on glucose to fuel aerobic glycolysis for the increased production of cellular metabolites required for generation of new biomass and to facilitate nutrient signaling.
Why do cancers have high aerobic glycolysis?
In summary, we suggest that upregulation of glycolytic metabolic pathways in the vast majority of invasive cancers is the result of adaptation to consistent environmental pressures in pre-malignant lesions, when diffusion limitations result in gradients of hypoxia and acidosis.
What happens when glycolysis is inhibited?
When glycolysis is inhibited, the intact mitochondria in normal cells enable them to use alternative energy sources such as fatty acids and amino acids to produce metabolic intermediates channeled to the TCA cycle for ATP production through respiration.
Why is rate of glycolysis higher in cancer cells?
Although glycolysis is less efficient than oxidative phosphorylation in the net yield of adenosine triphosphate (ATP), cancer cells adapt to this mathematical disadvantage by increased glucose up-take, which in turn facilitates a higher rate of glycolysis.
Which one of the following condition inhibit glycolysis?
In glycolysis, one of the end products is energy in the form of ATP. ATP acts as an inhibitor of phosphofructokinase-1, one of the main rate limiting enzymes in glycolysis.
What disease affects glycolysis?
Glycolysis is the conversion of glucose to pyruvate or lactate which results in the generation of ATP and has been shown to be abnormal in peripheral cells in Alzheimer's disease, Parkinson's disease, and Amyotrophic Lateral Sclerosis.
Does citrate inhibit glycolysis?
For example, citrate directly inhibits the main regulators of glycolysis, phosphofructokinase-1 (PFK1) and phosphofructokinase-2 (PFK2) [2,3], while it enhances gluconeogenesis by promoting fructose-1,6-biphosphatase (FBPase) [4].
What are the roles of glycolysis in macromolecular biosynthesis?
Apart from providing cellular energy, the metabolic intermediates of glycolysis also play a pivotal role in macromolecular biosynthesis, thus conferring selective advantage to cancer cells under diminished nutrient supply.
What is altered energy metabolism?
Altered energy metabolism is a biochemical fingerprint of cancer cells that represents one of the "hallmarks of cancer". This metabolic phenotype is characterized by preferential dependence on glycolysis (the process of conversion of glucose into pyruvate followed by lactate production) for energy production in an oxygen-independent manner.
Is glycolysis more efficient than oxidative phosphorylation?
Although glycolysis is less efficient than oxidative phosphorylation in the net yield of adenosine triphosphate (ATP), cancer cells adapt to this mathematical disadvantage by increased glucose up-take, which in turn facilitates a higher rate of glycolysis.
Is ATP a determinant of chemoresistance?
Accumulating data also indicate that intracellular ATP is a critical determinant of chemoresistance. Under hypoxic conditions where glycolysis remains the predominant energy producing pathway sensitizing cancer cells would require intracellular depletion of ATP by inhibition of glycolysis.
Is glycolysis a target for cancer?
Tumor glycol ysis as a target for cancer therapy: progress and prospects. Altered energy metabolism is a biochemical fingerprint of cancer cells that represents one of the "hallmarks of cancer". This metabolic phenotype is characterized by preferential dependence on glycolysis (the process of conversion of glucose into pyruvate followed by lactate ...
What is the glycolytic switch?
The glycolytic switch occupies a privileged position in the aggressive agenda of most solid tumors. Initially proceeding through suppression of the Pasteur Effect in response to hypoxia, it is indeed an early event marking the entry of dormant tumors into an exponential growth phase. As such, switching to a glycolytic metabolism may precede the evolution of tumors toward the more aggressive angiogenic and metastatic phenotypes. Glycolysis also exerts a pervasive influence throughout tumor growth, making of cancer a metabolic disease and suggesting necessary crosstalks between metabolism, angiogenesis, and metastasis. Persistence of high-rate glycolysis is under the master command of the transcription factor HIF-1 which, in collaboration with other oncogenic signaling pathways including c-Myc, AMPK, and mTOR, promotes the expression of most glycolytic enzymes and transporters. HIF-1 is inducible by hypoxia, thus bridging low pO 2 to the glycolytic phenotype for anaerobic energy production. But HIF-1 is also constitutively expressed in Warburg-phenotype tumor cells where it couples high-rate aerobic glycolysis to biosynthesis and cell proliferation. Solid tumors are the result of metabolic selection and a peculiar environment hosting different populations of metabolically overactive cells among which cells with aerobic glycolysis, anaerobic glycolysis and more oxidative phenotypes may cohabit. Our identification of a metabolic symbiosis based on the exchange of lactate between glycolytic and oxidative tumor cells provides a cooperative dimension ( Sonveaux et al., 2008 ), recently extended to non-malignant, supportive stromal cells ( Bonuccelli et al., 2010 ). Metabolism is not static but rather highly adaptive to external influences. The Warburg-phenotype itself is often reversible, as it is for non-malignant cells. The comprehensive (and therefore simplified in its expression) review that we provide here positions tumor metabolism as a key contributor to malignancy and as an attractive target for therapy. What have we learned until know? Researches worldwide have essentially shown that several regulators of glycolysis are amenable for anticancer therapy. The field is still in its infancy, though. Essential questions remain unanswered, as for example regarding the plastic adaptation of tumor metabolism to therapy. Furthermore, although many strategies have been being developed, some of which are currently evaluated in Phase I and Phase II clinical trials, there is still no grounded rationale to select one or several regulator (s) of glycolysis as preferred anticancer target (s) and little clinical information about toxicities. Should the therapy be tailored to a given tumor in a given patient? On which bases? One element to take into account could be the genetic background of the tumor based on experimental evidences that showed different metabolic outcomes deriving from some specific mutation ( Cairns et al., 2011 ). Systemic therapies directly targeting hypoxic tumor cells are generally confronted with difficulties to access to the hypoxic tumor cell compartment remote from blood vessels and to the emergence of resistance due to hypoxia-selected DNA instabilities. It is not clear whether antimetabolic therapies will do better than chemotherapy in this prospect. Alternatively, targeting metabolic cooperativity, which will necessitate a thorough understanding of the functions, regulations, and crosstalks between metabolic transporters, is an avenue recently opened for therapy.
What is the metabolic equation of cancer?
The first metabolic equation of cancer refers to energy production. It directly calls into play oxygen as the electron acceptor allowing the proper functioning of the respiratory chain at the inner mitochondrial membrane. Most solid tumors are hypoxic with many biological features accounting for the lack of oxygen ( Bristow and Hill, 2008 ). Probably the most comprehensive form of hypoxia is the so-called diffusion-limited hypoxia which arises when cells located at increasing distance from blood vessels eventually fail to receive the minimum amount of oxygen that they would need for an optimal oxidative metabolism in addition to the many non-metabolic redox reactions requiring oxygen ( Horsman and Overgaard, 2002 ). Normal cells in non-malignant tissues are also exposed to various levels of oxygen with respect to their distance from the closest blood vessel and owing to the fact that intermediate layers of cells consume oxygen. Evolution has selected the Pasteur Effect as a system aimed to finely tune cell metabolism in function of the local partial pressure of oxygen (pO 2; Wu and Racker, 1959 ). It relies on the negative feed-back exerted allosterically by energy metabolites [glucose-6-phosphate (G6P), citrate, and ATP] on key glycolytic enzymes, thus accelerating the glycolytic flux when the rate of oxidative phosphorylation (OXPHOS) decreases, and, inversely, improving the coupling between glycolysis and OXPHOS fluxes when oxygen levels increase (Figure 1 ). Two fundamentals of the Pasteur Effect are that glycolysis is nominally a faster succession of reactions than ATP production through OXPHOS ( Curi et al., 1988 ), and that the ATP yield of OXPHOS is nominally 19-fold higher than that of glycolysis alone. The full oxidation of one molecule of glucose provides up to 38 ATP molecules, whereas glycolysis alone provides only 2 ATP. Although theoretically OXPHOS is the best energy provider, the physiological reality is that both glycolysis and OXPHOS collaborate to produce ATP at a relative level dictated by local oxygen concentration. Tumor hypoxia is an extreme situation under which glycolysis becomes the main source of ATP in tumor cells ( Dang and Semenza, 1999 ). This glycolytic switch, formally corresponding to uncoupling glycolysis from OXPHOS, initially depends on repression of the Pasteur Effect. In this review, unless stated otherwise, the term “glycolysis” refers to “glycolysis uncoupled from OXPHOS of the tricarboxylic acid (TCA) cycle.” Direct molecular consequences are net increases in glucose consumption and lactic acid release (Figure 1 ). Because switching to glycolysis primarily reflects the metabolic adaptability of tumor cells to extreme environments, it is not surprising that lactate levels positively correlate with the aggressiveness of several types of human cancers ( Walenta et al., 1997, 2000, 2004; Brizel et al., 2001; Walenta and Mueller-Klieser, 2004 ).
What is the role of LDH5 in cancer?
By restoring the NAD + pool required for the GAPDH reaction, LDH5 plays an essential role in the perpetuation of high-rate glycolysis and is therefore recognized as a therapeutic target for cancer ( Xie et al., 2009; Le et al., 2010 ). High blood and tissue levels are associated with bad prognosis for many types of tumors (discussed in Koukourakis et al., 2011 ). The significance of LDH5 as a therapeutic target has been documented in recent studies showing that inhibition of its expression using RNA interference impairs tumor initiation, maintenance and progression ( Fantin et al., 2006; Le et al., 2010 ). A selective (with respect to LDH1 and GAPDH) competitive (with respect to NADH binding) inhibitor of LDH5, 3-dihydroxy-6-methyl-7- (phenylmethyl)-4-propylnaphthalene-1-carboxylic acid (FX11), has been identified through screening a bank of compounds derived from the natural product gossypol, a known malarial LDH inhibitor ( Yu et al., 2001b ). FX11 was recently shown to induce oxidative stress and cell death in vitro, which translated in vivo into inhibition of the progression of human lymphoma and pancreatic cancer xenografts ( Le et al., 2010 ). Although gossypol/AT-101 either alone or in association with chemotherapy is undergoing several Phase I and II clinical trials 2, the use of FX11 in clinical studies has not yet been reported. Recently, N -Hydroxy-2-carboxy-substituted indole compounds have been identified as LDH5-specific inhibitors ( Granchi et al., 2011 ). This series of compounds acts as competing inhibitors of LDH5 with respect to both NADH and pyruvate.
What is PKM2 inhibitor?
PKM2 is a master switch orienting glycolysis to ATP synthesis or to the production of biosynthetic blocks , making it an attractive target for anticancer treatments. In 2007, Thallion Pharmaceuticals started a Phase II clinical trial with the PKM2 inhibitor TLN-232/CAP-232, a seven amino-acid peptide administered to patients with refractory metastatic renal cell carcinoma. Encouraging results were reported in a poster displayed at the 33rd congress of the European Society for Medical Oncology in September 2008: two out of the 3 patients having completed the study showed stable disease and TLN-232 was generally safe and well tolerated. Recruitment for a second Phase II trial in metastatic melanoma patients started mid 2008 but was halted for legal reasons in June 2010 1. In 2010, Cantley’s lab screened a huge library of compounds to ultimately identify two water-soluble small molecule inhibitors with selectivity for PKM2 versus PKM1 ( Vander Heiden et al., 2010 ). Both molecules were reported to presumably block the allosteric F1,6BP-binding site of PKM2 absent in PKM1. Recently, shikonin and its enantiomeric isomer alkannin have been shown to inhibit PKM2 at concentrations that resulted in over 50% inhibition of PKM2 without affecting the activities of PKM1 and pyruvate kinase-L (harboring the same F1,6BP-binding site as PKM2; Chen et al., 2011 ). Both compounds inhibited glucose consumption and lactate release in MCF-7 and A549 tumor cells. These studies collectively demonstrate the possibility to identify specific PKM2 inhibitors that could serve as potent anticancer drugs. Interestingly, a recent publication describes the identification of PKM2 activators intended to be used as antiproliferative agents ( Boxer et al., 2010 ). These drugs would act as oncostatics.
What is the role of lactate in the tricarboxylic acid cycle?
In the presence of oxygen, lactate is oxidized to pyruvate by lactate dehydrogenase 1 (LDH1) and pyruvate fuels the tricarboxylic acid (TCA) cycle to produce ATP. The metabolic preference of oxidative tumor cells for lactate allows hypoxic tumor cells to get access to high levels of glucose.
Is cancer a metabolic disease?
Cancer is a metabolic disease and the solution of two metabolic equations: (i) to produce enough energy to survive when supplies and waste disposal are limited, and (ii) to divert enough metabolic intermediates from energy production to the bio synthetic pathways supporting cell proliferation. This review paper summarizes ...
What is therapeutic selectivity in cancer?
Therapeutic selectivity, or preferential killing of cancer cells without significant toxicity to normal cells, is one of the most important considerations in cancer chemotherapy. Understanding the biological differences between normal and cancer cells is essential for the design and development of anticancer drugs with selective anticancer activity. In the recent years, tremendous progress has been made in our understanding of the molecular mechanisms of cancer, in identification of specific genes and signaling pathways involved in carcinogenesis and cancer progression, and in developing chemical compounds or specific antibodies that specifically target the oncogenic molecules. Such target-specific agents have major advantages over the traditional chemotherapeutic compounds in that the targeting agents specifically interact with the key molecular players in cancer cells and have low toxicity to the normal cells. New agents with a high degree of target specificity and clinical therapeutic activity, exemplified by Gleevec (imatinib), Iressa (gefitinib), herceptin (trastuzumab), and rituximab, represent an exciting direction for cancer drug development. However, the mechanisms underlying cancer development and the disease progression are extremely complex, and it is now recognized that in many types of cancers there are multiple genetic and epigenetic alterations. Even within a specific cancer type, the malignant cell populations are heterogeneous and contain diverse genetic changes, which further alter over time because of genetic instability as the disease progresses. As such, it would be difficult to specifically kill these cancer cells by targeting a single gene. Proper combination of multiple target-specific agents may be required to effectively eliminate the entire cancer cell population. An alternative strategy to achieve both therapeutic selectivity and efficiency is to take advantage of the fundamental difference between cancer cells and normal cells in their biochemical metabolism. One of the most prominent metabolic alterations in cancer cells is the increase in aerobic glycolysis and the dependency on glycolytic pathway for ATP generation, known as the Warburg effect ( Warburg et al., 1924; Warburg, 1930, 1956 ). As this metabolic alteration is frequently seen in cancer cells of various tissue origins, targeting the glycolytic pathway may preferentially kill the malignant cells and likely have broad therapeutic implications. This review article will summarize several important aspects of the glycolytic pathway in cancer, compounds that inhibit glycolysis and other relevant metabolic processes, and their potential applications in cancer treatment.
How does a gene transfection affect glycolysis?
Studies using gene transfection approaches have revealed an intriguing possible mechanism by which malignant transformation by oncogenic signals may regulate energy metabolic pathways and renders the cancer cells highly glycolytic and become addictive to glycolysis for ATP production. Early studies in rodent cells showed that transfection with ras or src oncogenes led to a marked increase in the glucose uptake, accompanied by an increase in the expression of glucose transporter at both the mRNA and protein levels ( Flier et al., 1987 ). In embryotic cells, H-ras was shown to stimulate glycolysis and inhibits oxygen consumption ( Biaglow et al., 1997 ). The important role of Ras in promoting glycolysis was recently demonstrated in a study, in which transformation of cells by hTERT, SV-40T/t, and H-ras caused an increase in glycolysis dependency, and as the cells progressed toward a more tumorigenic state, they became more sensitive to the glycolytic inhibitor 2-DG ( Ramanathan et al., 2005 ). Interestingly, inhibition of H-ras by trans -farnesylthiosalicylic acid resulted in inhibition of glycolysis and cell death in human glioblastoma U87 cells, with concomitant decrease in HIF-1 α and glycolytic enzymes ( Blum et al., 2005 ).
What is the BCR-ABL oncogene?
The Bcr-Abl oncogene is a fusion DNA sequence created by chromosome translocation and codes for a constitutively active tyrosine kinase fusion protein. The BCR-ABL-positive cells express the high-affinity glucose transporter (GLUT-1) and exhibit increased glucose uptake. Imatinib treatment decreased the activity of both hexokinase and glucose-6-phosphate dehydrogenase (G6PD) in leukemia cells, leading to suppression of aerobic glycolysis ( Boren et al., 2001; Gottschalk et al., 2004; Serkova and Boros, 2005 ). A decrease in G6PD activity would lead to lower glucose flow into the pentose phosphate pathway, and thus deprives transformed cells of metabolic intermediates for ATP generation and substrates for macromolecule synthesis. Although imatinib is an antileukemia drug, its ability to suppress aerobic glycolysis may make it useful for the treatment of certain solid tumors.
Why do cancer cells need more glucose?
Because ATP generation via glycolysis is far less efficient (two ATP per glucose) than through oxidative phosphorylation (36 ATP per glucose), cancer cells consume far more glucose than normal cells to maintain sufficient ATP supply for their active metabolism and proliferation. As such, maintaining a high level of glycolytic activity is essential for cancer cells to survive and growth. This metabolic feature has led to the hypothesis that inhibition of glycolysis may severely abolish ATP generation in cancer cells and thus may preferentially kill the malignant cells ( Munoz-Pinedo et al., 2003; Izyumov et al., 2004; Xu et al., 2005b ). As illustrated in Figure 2, under physiological conditions, normal cells with intact mitochondrial function can effectively use glucose and other metabolic intermediates to generate ATP through the TCA cycle and oxidative phosphorylation in the mitochondria (green arrows). However, the ability of cancer cells to use the mitochondrial respiratory machinery to generate ATP is compromised for the reason described above. This forces the cancer cells to increase their glycolytic activity to maintain sufficient ATP generation. It is postulated that such a metabolic adaptation eventually renders cancer cells highly addictive to and dependent on the glycolytic pathway (red arrows), and become vulnerable to glycolytic inhibition ( Gatenby and Gillies, 2004; Xu et al., 2005b ). When glycolysis is inhibited, the intact mitochondria in normal cells enable them to use alternative energy sources such as fatty acids and amino acids to produce metabolic intermediates channeled to the TCA cycle for ATP production through respiration. As such, cells with normal mitochondria are expected to be less sensitive to agents that inhibit glycolysis.
How much ATP is produced in oxidative phosphorylation?
Because the production of ATP is much more efficient through oxidative phosphorylation (36 ATP per glucose) than by glycolysis (two ATP per glucose), a small loss of respiratory function would require a substantial increase of glycolytic activity to maintain the energy balance.
What is the role of G-6-P in gluconeogenesis?
The interconversion of G-6-P and fructose-6-phosphate is catalysed by phosphoglucose isomerase (PGI), which plays an important role in both the glycolytic and gluconeogenesis pathways ( Harrison, 1974 ). Interestingly, recent studies have revealed that GPI can also function as an autocrine motility factor (AMF), which is secreted from the tumor cells to promote cell motility and proliferation ( Niinaka et al., 1998; Sun et al, 1999 ). Autocrine motility factor and its receptor AMFR (gp78) were originally identified in melanoma and oncogene-transfected metastatic NIH3T3 cells, and the AMF/AMFR interaction seems to stimulate tumor cell migration in vitro and enhance metastasis and angiogenesis in vivo ( Liotta et al, 1986; Nabi et al., 1990; Watanabe et al., 1996; Funasaka et al., 2001, 2002 ). Autocrine motility factor receptor is overexpressed in various metastatic tumors and is correlated with a poor prognosis ( Hirono et al., 1996 ). The presence of GPI in serum and urine is associated with cancer progression and indicates poor prognosis ( Baumann and Brand, 1988; Baumann et al., 1990; Filella et al., 1991 ). The expression of PGI is stimulated by hypoxia ( Yoon et al., 2001; Niizeki et al., 2002 ). Thus, in addition to its well-defined enzymatic activity in the glycolytic pathway, GPI also functions as a cytokine extracellularly and is associated with aggressive malignant behaviors.
Does aerobic glycolysis increase in cancer cells?
The phenomenon of aerobic glycolysis increase in cancer cells was first described by Otto Warburg (1930) over 70 years ago. He showed that compared to normal cells, malignant cells exhibit significantly elevated glycolytic activity even in the presence of sufficient oxygen, and considered this phenomenon as the most fundamental metabolic alteration in malignant transformation, or ‘the origin of cancer cells’ ( Warburg, 1956 ). Although the cause–effect relationship between the increase in aerobic glycolysis and the development of cancer is controversial ( Zu and Guppy, 2004 ), increased glycolysis has been consistently observed in many cancer cells of various tissue origins (for a review, see Semenza et al., 2001 ), suggesting that this metabolic alteration is common in cancer. Indeed, the positron emission tomography (PET) widely used in clinical diagnosis of cancer is based on the fact that cancer cells are highly glycolytic and actively uptake glucose. The Warburg effect can be viewed as a prominent biochemical symptom of cancer cells that reflects a fundamental change in their energy metabolic activity. Several mechanisms have been suggested to affect energy metabolism and thus contribute to the Warburg effect. These mechanisms include (1) mitochondrial defects, (2) adaptation to hypoxic environment in cancer tissues, (3) oncogenic signals, and (4) abnormal expression of certain metabolic enzymes. Table 1 provides a summary and explanations of these possible mechanisms.
What is the role of glycosylation in cancer?
Understanding the changes that occur in neoplastic cells and their interactions may be the key to rational drug design. Among the major changes, alterations in the glycosylation process are a well-established hallmark of cancer [ 3 ]. Glycosylation is the enzymatic process responsible for the attachment of glycans (carbohydrates) to proteins, lipids, or other saccharides ( Figure 1 ). This major post-translational modification (PTM) occurs in the endoplasmic reticulum/Golgi compartment of essentially all cells and it is mediated by the coordinated action of a portfolio of different glycosyltransferase and glycosidase enzymes that, in a series of steps, form carbohydrate structures [ 3, 4 ]. It is comparable with phosphorylation in terms of the range of modified sites and certainly exceeds it in terms of complexity and structural diversity.
Which atom is the glycan linked to?
glycans covalently linked to a polypeptide via an oxygen atom through serine (Ser) or threonine (Thr) residues.
What is the enzymatic process of glycosylation?
Schematic Representation of Glycosylation Diversity. The glycosylation process is a well-orchestrated enzymatic process of building sugar structures: glycans. Glycans can be attached to a variety of molecules such as proteins (glycoproteins) or lipids (glycolipids) or exist as free structures, such as hyaluronic acid.
Why is doxorubicin resistant to chemo?
For instance, resistance to the chemotherapeutic agent doxorubicin (DXR), a front-line treatment for some type of cancers, is due to glycosylation at cell surface proteins, which may affect epitope accessibility and drug binding to receptor proteins [ 30 ].
How many antibodies are there for cancer?
Antibodies are now an established option in cancer treatment, with more than 30 antibodies or antibody derivatives currently approved by the FDA and European Medicines Agency and hundreds more in clinical trials [ 48 ]. Anticancer mAbs target a broad range of antigens, including soluble proteins, cancer cell surfaces, and effector cell receptors. Such versatility allows mAbs to exploit several mechanisms of action, including the direct blocking of growth factors, the interruption or induction of signaling pathways, and the modulation of the immune system [ 48, 49 ]. MAbs as therapeutic drugs offer some advantages, such as relatively high specificity, low toxicity, and long half-life (i.e., weeks) in the human circulation. They have also disadvantages, such as the laborious production process undergoing the relatively high heterogeneity due to, for example, post-translational modifications (PTMs). However, these modifications offer opportunities to include additional functionalities and thereby provide possibilities to further fine-tune the efficacy of some therapeutic antibodies [ 50, 51 ].
Why is automatization used in drug discovery?
automatized methodology vastly used in drug discovery due to capacity to test a large number of synthetic compounds in miniaturized in vitro assays to identify those capable of modulating the biological target of interest.
What happens when a tumor is detected in an advanced stage?
Unfortunately, the majority of tumors are detected in an advanced stage, leading to treatment failure . This therapeutic failure often results in tumor recurrence and metastasis, which accounts for approximately 90% of cancer deaths [ 1 ].
What are some natural compounds that affect the expression of glucose transporters?
Many natural compounds affect the expression of glucose transporters (especially GLUT1 and GLUT4) indirectly. Flavones, polyphenols, and alkaloids are interesting bioactive anticancer molecules isolated from plants, as several of them have been repeatedly reported to control glucose transporter activity in different cancer cell models. ...
What is the term for the metabolic pathway where cells take glucose?
As a young scientist in the 1920s, Otto Heinrich Warburg described an elevated rate of glycolysis occurring in cancer cells, even in the presence of atmospheric oxygen (the Warburg effect) that earned him a Nobel Prize. Glycolysis is the metabolic pathway where cells take glucose. A type of sugar; the chief source of energy for living organisms.
What is the pathway of glucose?
Glycolysis is the metabolic pathway where cells take glucose. A type of sugar; the chief source of energy for living organisms. to produce energy for cell function and replication. [1] The alternative cancer world has long seen this fact as a therapeutic strategy to help block cancer’s fuel source. Let's explore the latest scientific data ...
What is the enzyme that inhibits HK?
Methyl jasmonate, a plant stress hormone produced by many plants including rosemary, olive, and ginger, inhibits HK, triggering apoptosis in cancer cells. [11] Another enzyme, pyruvate kinase, is specifically expressed in cancer cells and plays an important role in the metabolism and replication. [12] .
Does green tea inhibit LDH?
EGCg from green tea extract has been recently shown to have LDH-A inhibiting activity. [18] But other natural compounds, such as furanodiene and maslinic acid (found in curcumin, ginger, and olive oil derivatives) could increase the LDH release in cancer cells by inducing cancer cell injury. [19, 20]
Can natural compounds help with glycolysis?
If there exist natural compounds to help regulate these glycolysis-signaling pathways, we may help more people with cancer. [3, 4] First, let’s explore nutrients that may regulate the glucose transporters that are related to glycolysis. Many natural compounds affect the expression of glucose transporters (especially GLUT1 and GLUT4) indirectly.
Does HIF-1 increase glucose?
It up-regulates the glucose transporters (GLUT) that increase the amount of glucose getting into the cell, a bad thing for those with cancer as it fuels the fire. It induces the expression of glycolytic enzymes, such as hexokinase, pyruvate kinase, and lactate dehydrogenase, so glucose is more readily used as an energy source. If there exist natural compounds to help regulate these glycolysis-signaling pathways, we may help more people with cancer. [3, 4]

Glycolysis and Cancer Bioenergetics
Glycolysis and Biosynthesis in Cancer
- The second metabolic equation of cancer refers to the biosynthesis of cell constituents. Glucose is a major source of carbohydrates, with as direct consequence that full energy extraction in oxidative pathways would deprive cells from important biosynthetic blocks. Similarly, a glycolytic cell producing lactate from glucose stoichiometrically would fail to proliferate. The second meta…
Anticancer Targets in The Glycolytic Metabolism of Tumors
- The aforementioned observations position glycolysis as a key contributor to the malignant phenotype and support the quest for new anticancer treatments targeting glycolysis. Indeed, most of the molecular adaptations supporting high-rate glycolysis are either unique to cancer in an otherwise healthy organism or druggable with manageable toxicities. ...
Concluding Remarks
- The glycolytic switch occupies a privileged position in the aggressive agenda of most solid tumors. Initially proceeding through suppression of the Pasteur Effect in response to hypoxia, it is indeed an early event marking the entry of dormant tumors into an exponential growth phase. As such, switching to a glycolytic metabolism may precede the evolution of tumors toward the mor…
Conflict of Interest Statement
- The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
- Works at the authors’ lab are supported by the European Research Council (FP7/2007-2013 ERC Independent Researcher Starting Grant 243188 TUMETABO to Pierre Sonveaux), the Belgian Fonds National de la Recherche Scientifique (F.R.S.-FNRS), the Communauté Française de Belgique (ARC 09/14-020), and the Fondation Belge Contre le Cancer(200-2008). Paolo E. Porpor…