What are the new drugs available to treat hepatitis C?
Jun 11, 2021 · Download PDF 0.5MB. Share. Sovaldi, a direct-acting antiviral (DAA) treatment for hepatitis C virus (HCV), had an average wholesale acquisition cost (WAC) of $1,000 per day in 2013, or $84,000 for a 12-week course of treatment. Sovaldi’s launch kicked off the second generation of DAAs for HCV, which expanded more widely tolerable treatment options to …
How has hepatitis C treatment evolved?
Nov 22, 2013 · The wholesale acquisition cost (WAC) for simeprevir is $790 per 150 mg capsule. The cost for a 28-days supply of simeprevir is $22,120 and a 12-week supply is $66,360. Thus, a typical 12-week treatment course of simeprevir when used with a total of 24-weeks of peginterferon plus ribavirin will cost approximately $85,000.
Are cost-effectiveness evaluations for hepatitis C virus treatments becoming obsolete?
Jun 01, 2018 · The table below highlights the average cost of treatment for the combination DAAs currently available. Most of these drugs take at least 12 weeks to cure HCV, while the most recently approved drug ...
Are new hepatitis C drugs a financial burden for Medicare Part D patients?
Jun 16, 2016 · Objective. We aimed to determine the association between the stepwise increase in the sustained viral response (SVR) and Swiss and United States (US) market prices of drug regimens for treatment-naive, genotype 1 chronic hepatitis …
What is the cost of hep C treatment?
A 2018 study found that a single pill of one hepatitis C drug cost $1,000. The total was $84,000 for its 12-week course of treatment. Another drug cost $23,600 per month. That's for treatment that could take 6 months to a year.Jun 26, 2020
Are hep C drugs expensive?
Hepatitis C drugs are pricey Antiviral drugs for hepatitis C are very effective, but they come at a steep cost. Just one Sovaldi pill costs $1,000. A full 12-week course of treatment with this drug costs $84,000.Feb 5, 2019
How much do direct-acting antivirals cost?
Conclusions: Within the next 15 years, large-scale manufacture of 2 or 3 drug combinations of HCV DAAs is feasible, with minimum target prices of $100-$250 per 12-week treatment course. These low prices could make widespread access to HCV treatment in low- and middle-income countries a realistic goal.
How much does Simeprevir cost?
Cost and Medication Access The cost for a 28-days supply of simeprevir is $22,120 and a 12-week supply is $66,360. Thus, a typical 12-week treatment course of simeprevir when used with a total of 24-weeks of peginterferon plus ribavirin will cost approximately $85,000.
What is the best hep C treatment?
Hepatitis C is treated using direct-acting antiviral (DAA) tablets. DAA tablets are the safest and most effective medicines for treating hepatitis C. They're highly effective at clearing the infection in more than 90% of people.
Does hep C qualify for disability?
An individual with hepatitis C may be eligible for disability income if they meet the requirements outlined in the SSA's Listing of Impairments under Section 5.05, titled “Chronic liver disease.” Learn about the symptoms of chronic hepatitis C.Oct 28, 2021
Is Hep C curable 2020?
Hepatitis C (hep C) infection used to be a lifelong condition for most people. Up to 50 percent of people may clear the hepatitis C virus (HCV) from their body without treatment. For everyone else, the infection becomes chronic. With advances in hep C treatment, most people can now be cured of HCV.
How much is hep C treatment in India?
The generic version of these drugs are available in cities such as Bengaluru Hyderabad and Chennai at the cost of Rs70000 or around $1000 USD for the entire treatment regimen.
How much does hep C treatment cost UK?
A 12-week course of treatment with elbasvir-grazoprevir usually costs £36,500 per patient, but the NHS will pay less than this as the company has offered a confidential discount. Taken once daily, the tablet could treat around 4,000 patients in the first year, alongside other options already available for hepatitis C.
Why was simeprevir discontinued?
— The discontinuation was not due to any safety, efficacy or quality issues. — Moreover, Janssen will voluntary withdraw the New Drug Application for Olysio in the U.S. and product will no longer be available, effective May 25, 2018.
Does insurance cover hep C drugs?
Not all health insurance plans cover all prescribed medications for HCV treatment with few exceptions. Most insurers cover Sovaldi. It has an estimated copay of $75 to $175 per month. Check with your insurance provider to see what your individual coverage may entail.
Does insurance cover hep C treatment?
Luckily, hep C treatment is covered by most insurance plans, so for many people, the cheapest way of getting it will be through insurance (although you'll probably need prior authorization). If your hep C treatment is not covered by your insurance, ask your doctor about an appeal.Jan 27, 2019
What is the best treatment for hepatitis C genotype 1?
Simeprevir is indicated as a component of combination antiviral treatment for patients with chronic hepatitis C genotype 1 infection. Simeprevir is approved for use with (a) peginterferon-alfa and ribavirin or (b) in combination with sofosbuvir.
When was Simeprevir approved?
FDA Status. Simeprevir was approved by the U.S. FDA on November 22, 2013 for the treatment of individuals with genotype 1 chronic hepatitis C as a component of combination therapy with peginterferon-alfa and ribavirin. On November 5, 2014, the U.S. FDA approved the use of simeprevir in combination with sofosbuvir for patients with genotype 1 ...
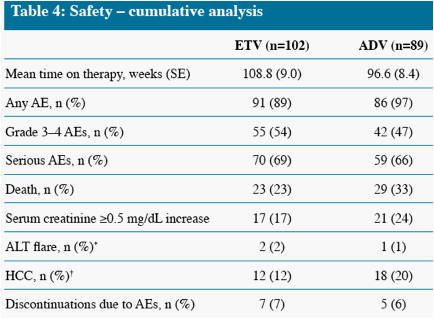
What are the side effects of simeprevir?
Adverse Effects. The most common adverse effects attributable to simeprevir are rash (including a potentially serious photosensitivity reaction), pruritus, and nausea. Figure 3. Simeprevir Rash. Pruritic rash on hands of patient that started 2 weeks into treatment with simeprevir plus sofosbuvir.
What is the simeprevir?
Simeprevir is a NS3/4A hepatitis C virus (HCV) protease inhibitor. Simeprevir is a macrocyclic compound that non-covalently binds to and inhibits the NS3/4A HCV protease, a protein that is responsible for cleaving and processing the HCV-encoded polyprotein, a critical step in HCV viral life cycle. Simeprevir is considered a second generation HCV ...
Where is simeprevir metabolized?
Simeprevir is metabolized primarily in the liver and patients with moderate or severe hepatic impairment (Child-Pugh Class B or C) would be expected to have higher levels of simeprevir. No dosing recommendation for simeprevir is given for patient with moderate to severe hepatic impairment.
Is Simeprevir a protease inhibitor?
Simeprevir is considered a second generation HCV protease inhibitor because of the enhanced binding affinity and specificity to NS3/4A when compared with the first-generation protease inhibitors with linear structure.
Does simeprevir cause rash?
Rash not related to photosensitivity can also occur and similar to the photosensitivity rash most often develops during the first 4 weeks of therapy. Simeprevir contains a sulfonamide moiety, but insufficient data exist to know the risk of taking simeprevir in persons with a prior "sulfa allergy".
How many people die from hepatitis C each year?
Americans have chronic hepatitis C. About 19,000 of these people die each year from cirrhosis or liver cancer. Fortunately, recent advancements in the fight against this virus have changed the outlook for people with HCV. New drugs have transformed the disease from one that can, at best, be controlled to one that can be cured for most people who ...
How to pay for HCV?
If you’re concerned about paying for HCV medications, remember that you aren’t alone as you seek treatment. There are people and organizations that can help you, including the following: 1 Your doctor. They can help you by ordering and documenting the tests you’ll need so you can qualify to get your medications, especially if you’re working with a liver or infection specialist. 2 Most drug manufacturers. There are patient assistance programs that offer free or reduced-cost medications for people who meet their criteria. 3 Patient advocacy groups. These groups provide assistance with all aspects of HCV treatment. For instance, if your insurer denies treatment, you can appeal the decision with help from one of these groups. Your doctor can also help in this situation.
What is the liver infection?
Hepatitis C is a viral infection that attacks the liver. Infection with hepatitis C can lead to serious liver disease, including cirrhosis and cancer. Hepatitis C virus (HCV) is transmitted by exposure to blood or other bodily fluids that contain HCV.
What is a direct acting antiviral?
of people who take them, depending on the type of HCV infection and treatment exposure. These new drugs are called direct-acting antivirals (DAAs). The U.S. Food and Drug Administration (FDA) approved the first of these medications for HCV treatment in 2011. Several more medications have been approved since that time.
Is generic medicine cheaper than brand name?
It also means there are no generic versions of these drugs yet. Generics are typically much cheaper than brand- name versions. The FDA determines how long this period of exclusivity will last. During this time, the pharmaceutical companies have a lot of freedom in establishing prices.
What are the criteria for liver disease?
These criteria may be based on: the severity of liver disease. whether the person avoids alcohol and drug use. whether the drug’s prescribed by a doctor who specializes in liver diseases. the life expectancy of the person seeking treatment. whether less expensive treatments could be used first.
Can hepatitis C be treated with drugs?
Today there are several drug options available that can cure hepatitis C infection — that’s the great news. What’s less great is the high cost of these drugs. However, there are many options you can explore to find help paying for these medications.
How many people in the US have HCV?
More than 3 million Americans are infected with HCV, with its prevalence concentrated among baby boomers, who were born between 1945 and 1965. 7 HCV causes more deaths in the United States than HIV/AIDS. 8 Chronic HCV is a cause of serious and costly liver diseases, such as cirrhosis and liver cancer, and related hospitalizations and costs have increased during the past decade. 9 Although the burden of HCV can be reduced through screening and treatments, the implementation of recommended screening is limited, and half of the infected population goes undiagnosed. 9
What drugs did Part D cover?
All Part D plans covered 2 new HCV drugs, Olysio and Sovaldi, and 98% of plans covered Harvoni ( ). Only 33% of MAPDs and 30% of PDPs covered Viekira Pak. Nearly every plan that covered these new drugs used prior authorization and nearly half of the plans used quantity limits. Almost all plans placed new HCV agents in a specialty tier and required coinsurance rather than co-payment. The average coinsurance rate was slightly higher among MAPDs than PDPs (31.4% vs 28.7%), but it varied more among MAPDs (20%-50%) than PDPs (25%-33%).
What is Medicare Part D?
Medicare Part D provides outpatient prescription drug coverage to the elderly and disabled. It is delivered through private plans, including standalone prescription drug plans (PDPs) or Medicare Advantage plans with prescription drug coverage (MA-PDs). Medicare specifies a standard Part D benefit package, but plans can modify the benefits as long as their schemes are equal in value to the standard package.
Does Part D insurance cover HCV?
Part D plans charge relatively high coinsurance for new HCV drugs, and they require rigorous utilization management, including prior authorization and quantity limits for those drugs. Little variation in coverage exists across plans, leaving few options for beneficiaries to choose a plan with better benefits.
How old do you have to be to get tested for hepatitis C?
The CDC recommends testing for hepatitis C virus in all adults aged 18 years and older, as well as many other groups.
When was Epclusa approved?
Epclusa was first approved by the FDA in June of 2016. It is approved for use in adults and pediatric patients at least 3 years of age with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5, or 6 infection. Epclusa is used with ribavirin in patients with advanced liver disease (decompensated cirrhosis).
What are the side effects of Epclusa?
The most common side effects of Epclusa in adults and children 6 years and older include headache and tiredness. When used with ribavirin in advanced liver disease in adults, common side effects also include anemia (not enough red blood cells), upset stomach (nausea), trouble sleeping, and diarrhea. In children less than 6 years old, vomiting and ...
Is Epclusa a ribavirin?
Epclusa is used with ribavirin in patients with advanced liver disease (decompensated cirrho sis). In August 2017, the FDA also approved Epclusa to treat chronic HCV in patients co-infected with HIV. Gilead Science's Epclusa is a nucleotide analog polymerase inhibitor and pan-genotypic NS5A inhibitor fixed-dose combination for the treatment ...
Does Epclusa cure HCV?
Official Answer. Yes, Epclusa ( sofosbuvir and velpatasvir) can cure hepatitis C virus (HCV) in many patients and has a 98% overall cure rate in all 6 of the main types of hepatitis C. "Cure” means the Hep C virus is not detected in the blood when measured three months after treatment is completed. In studies, these cure rates were seen in patients ...
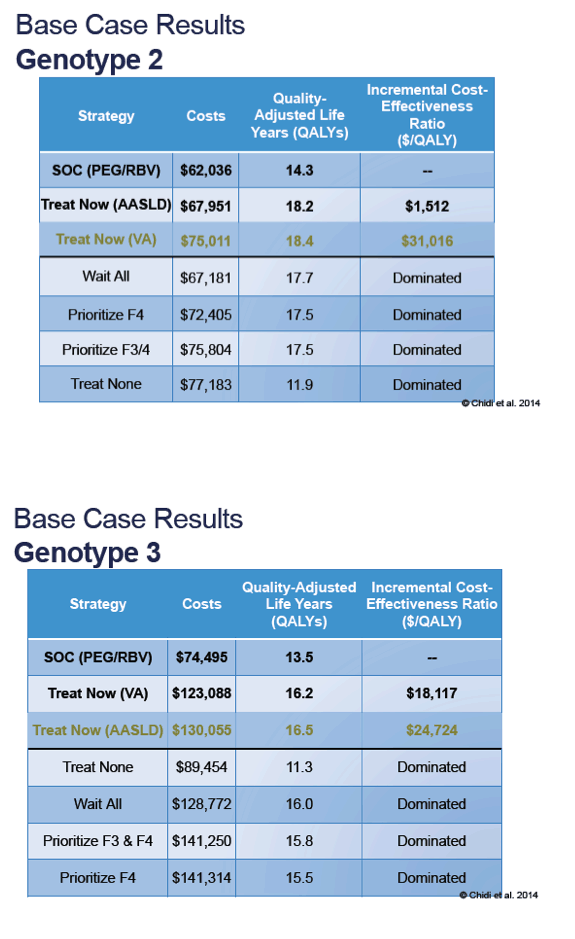
What is the primary analysis for this methodology study focused on?
The primary analysis for this methodology study focused on the changing costs and effectiveness estimates at each time point to estimate incremental cost-effectiveness ratios. A scenario analysis was conducted using only the WAC for each drug referenced in RED BOOK to describe the effect of using list versus net price in the CEA. 21
Is HCV treatment effective?
Treatment effectiveness for HCV has increased steadily, while treatment costs increased substantially from 2010-2014 before decreasing to its lowest point in 2018. The dynamic nature of CEAs in a disease state with rapid pharmaceutical innovation may cause some concern for decision makers who rely on a single analysis over time. Model transparency along with resources to update or revise model assumptions would enable organizations to provide more up-to-date results to inform formulary decisions.
How much does Sovaldi cost?
When Sovaldi entered the market, newspapers reported that Sovaldi cost $1,000 a day, or $84,000 per person for a 12 week treatment . Approximately a year later, when Harvoni and then Viekira Pak entered the market, their costs were reported respectively as $94,500 and $83,319 per person for a 12 week treatment.
Do PBMs disclose their deals?
Although virtually all PBMs claim to have negotiated significant “deals” with hepatitis C drug manufacturers, no PBMs have been willing to disclose the terms of their deals. However, during investor conference calls, hepatitis C drug manufacturers have revealed basic information about these drug deals.
