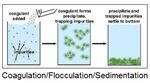
Coagulation
In water treatment, coagulation flocculation involves the addition of polymers that clump the small, destabilized particles together into larger aggregates so that they can be more easily separated from the water. Coagulation is a chemical process that involves neutralization of …
What is coagulation normal values?
The normal time is usually reported as less than 30 to 35 seconds depending on the technique used. In fact, there is a normal range of about 10 seconds (e.g., 25 to 35), and decreased values ("short") may also be abnormal. Basic Science
What are flocculants and coagulants for wastewater treatment?
Removal of Inorganics
- Arsenic removal. Arsenic is a commonly occurring toxic element and long term exposure to arsenic is injurious to health.
- Fluoride removal. In 1975, the EPA named fluoride as a contaminant in the National Interim Primary Drinking Water Regulations.
- Chemical Phosphorus Removal. ...
What does a coagulation test determine?
What Is A Coagulation Test? A coagulation test measures blood’s capability to clot and if it clots how long it does take to clot. This test helps the doctor to assess the risk of developing clots (thrombosis) or excessive bleeding in blood vessels. These tests are identical to many other blood tests and the risks and side effects are least.
What is the purpose of a coagulation test?
- non-homogenous: realization of the three-dimensional model of the clot growth
- use of platelet free plasma
- record of information about the clot formation as a diagram, giving the possibility to calculate the key parameters of the blood coagulation system
- new test, not widely accepted
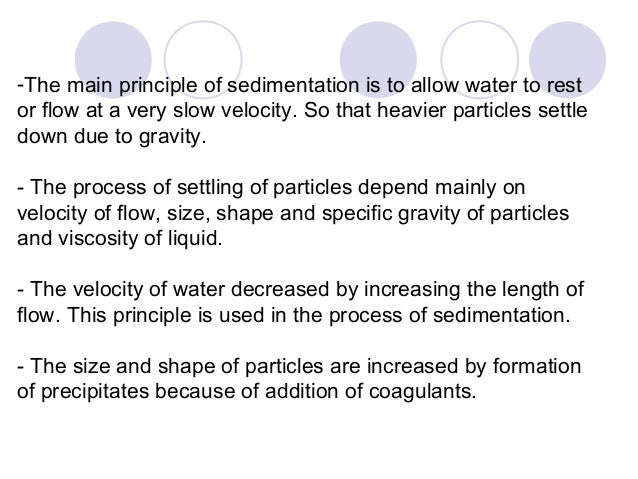
What is coagulation what coagulants are used in water treatment?
Chemicals (coagulants) are added to the water to bring the nonsettling particles together into larger, heavier masses of solids called floc. Aluminum sulfate (alum) is the most common coagulant used for water purification. Other chemicals, such as ferric sulfate or sodium aluminate, may also be used.
Why coagulation is so important for wastewater treatment?
Coagulation helps to remove a number of different pollutants that cause your water to become dirty or toxic, including: Organic compounds and certain dissolved organic materials, commonly referred to as Natural Organic Matter (NOM) or Dissolved Organic Carbon (DOC)
What is the role of a coagulant?
Coagulants and flocculation processes are used to remove colloidal impurities: suspended particles such as bacteria, clay, silts, and organic matter from the contaminated water. This produces large flock aggregates that can be removed from the water in subsequent clarification/filtration processes.
What is the principle of coagulation?
At a glanceWorking PrincipleSuspended particles are destabilised by addition of a clarifying agent leading to the neutralisation of their charges. Particles thus agglomerate (flocs formation) and are able to decant.Main strengthRemoves solids and improves filtrationMain weaknessContinuous input of chemicals required6 more rows•May 24, 2019
What is coagulation in water treatment PDF?
Coagulation is the destabilization and aggregation of a colloidal dispersion to permit particle removal by sedimentation and for filtration. The physical chemistry of coagulation may be considered as: 1.
What is the function of coagulation in water treatment quizlet?
The purpose of coagulation and flocculation is to remove particulate impurities and color from the water being treated.
What is coagulation in water?
Coagulation is a process for combining small particles into larger aggregates (flocs) and for adsorbing dissolved organic matter on to particulate aggregates so that these impurities can be removed in subsequent solid/liquid separation processes. The modern use of coagulants for water treatment started more than 100 years ago, when ferric chloride and aluminum sulfate were used as coagulants in full-scale water treatment works. The coagulation mechanism was firstly explained by the Schultz–Hardy rule and the Smoluchowski's particle collision function, which form the theoretic basis of coagulant demand and changes in particle number in flocculation process. Mattson [ 1] firstly derived that the hydrolysis products of Al and Fe salts were more important than the trivalent ions themselves, although this approach was widely accepted and accorded its proper position in coagulation chemistry 30 years later. Black and co-workers [ 2] conducted a series of studies on the effect of pH and various anions on the time of floc formation. After these early studies, the coagulation research focused on the study to produce better flocs and search for better coagulant aids including bentonite, silicates, and limestone.
Why is coagulation important?
Indeed, the performance of coagulation process is one of major factors in improving overall efficiency and cost effectiveness of water treatment. Future research needs are suggested to pay particular attention to the aspects of developing and using more effective coagulants/flocculants; optimizing unit configurations and process design, dose control and sludge handling; and more fundamental studies of the properties and behavior of coagulating chemicals.
What are the recent researches in coagulation areas?
Subsequent sections will introduce recent researches in coagulation areas, including (1) the development of new type of coagulants, especially the composite polymeric coagulants; (2) investigation on the characteristics of flocs developed in the coagulation/flocculation; (3) studies on the hybrid processes combining coagulation with other technologies for water and wastewater treatment, and (4) practical approaches on the control of coagulation.
How is coagulation controlled?
Coagulant is dosed into the raw water with pH adjustment by adding either acid or base. Both pH and SCD levels are monitored and controlled by separately feedback loops. This control strategy regulates the SCD signal and generates a value of SCD measurement, which is equivalent to that under the predetermined optimal pH value. This eliminates the effect of process pH on the SCD measurement, reduces controller interactions and provides a more robust coagulation control tool.
What are coagulants used for?
Coagulants used for water and wastewater treatment are predominantly inorganic salts of iron and aluminum. When dosed into water the iron or aluminum ions hydrolyse rapidly and in an uncontrolled manner, to form a range of metal hydrolysis species.
What was the first step in the development of a comprehensive theory of coagulation?
A significant step in the development of a comprehensive theory of coagulation during 1960s was the introduction of micro-electrophoresis [ 5] to the study of colloidal destabilization which allowed the quantification of electrical charge on colloidal particles. Study of the stoichiometric relationship between the coagulant dose required to neutralize the colloids and the concentration of colloidal impurities in water also started since that decade. These studies pointed out the effect of pH, ionic strength and the properties of pollutants on the removal efficiency of colloidal particle. These studies also re-emphasized the importance of hydrolysis products of the coagulants, as originally proposed by Mattson, and established an adsorption model to detail the coagulation mechanism of hydrolysed metal coagulants.
When did coagulants start being used?
Modern use of coagulants for water treatment started more than 100 years ago and researches on coagulation have been continuously undertaking since then. Fundamental and applied studies have derived comprehensive coagulation/flocculation theories, novel coagulant, advanced study tools, hybrid processes and practical experience, aiming at raising overall treatment efficiency and cost effectiveness and meeting the stringent water quality standards.
Why is coagulation important?
Coagulation plays primary and important roles in water and wastewater treatment. Researches are needed for novel coagulants, hybrid processes and control schemes. Study of floc properties is essential to enhance the coagulation efficiency.
What is the performance of coagulation?
The performance of coagulation is the major factor in improving water treatment efficiency. Water industries globally consider coagulation/flocculation is one of the major treatment units used to improve overall treatment efficiency and cost effectiveness for water and wastewater treatment.
What are Coagulation and Flocculation in Water Treatment?
Coagulation and flocculation are two processes that go together in water treatment. They are separate, but they are used one after the other to remove particles in water.
How Coagulation Water Treatment Works
Coagulation water treatment prevents the suspended particles from repelling one another and encourages them to form into clumps, or flocs.
How Flocculation Works
The flocculation process follows coagulation in water treatment. Coagulation is the charge neutralisation of fine particles, and flocculants are the agents that then promote the clumping of these particles together.
How Does Temperature Affect Coagulation in Water Treatment?
Temperature can have a significant effect on coagulation and flocculation.
Is Coagulation Caused by Bacteria in Water Treatment?
Suspended solids in water can be the result of natural causes, arising from organic materials such as algae, or inorganic materials such as sediment or silt.
How to Maximise the Effects of Water Treatment
Coagulation is a long-established water treatment, but it doesn’t remove all bacteria from water systems.
What is the role of coagulation in wastewater treatment?
Coagulation plays a vital role in the wastewater treatment process, allowing for solids removal and dewatering, water clarification, lime softening, and sludge thickening.
What is coagulation in water?
Through coagulation, industrial water supplies are put into the perfect chemical state for easy mechanical filtration. Once the flocs settle at the bottom of your clarifier, equipment like a filter press can then take those larger clumps of aggregated particles and remove them, delivering clean water back into your system.
What is the name of the cationic coagulant that neutralizes the negative charge of colloids in water?
PolyAMINEs and PolyDADMACs – These cationic coagulants work by charge neutralization alone and are the most widely used organic coagulants. PolyAMINEs and PolyDADMACs neutralize the negative charge of colloids in your water, forming a spongy mass called a “microfloc.” Since they only coagulate through charge neutralization, they don’t offer any advantages in regard to the sweep-floc mechanism (explained later with inorganic coagulants).
What is the process of adding coagulants to water?
These help to clean the water by absorbing impurities in the water as they fall. This process is known as the “sweep-floc” mechanism. However, this can add to the overall sludge volume that must be treated and removed, so it’s not the right choice in every scenario.
What is the purpose of coagulant?
The primary purpose of using a coagulant besides removing vary fine particles from suspension is that this process results also in less turbidity of the water, i.e. clearer water.
What happens when coagulants are positive?
With coagulants’ positive charge, the negatively charged particles in the water are neutralized. This causes the suspended solids in the water to bind together into larger flocs. These larger flocs begin to settle at the base of the water supply. The larger the size of the particles, the quicker the floc settles.
What is the biggest factor in selecting a coagulant?
The biggest factor in selecting a coagulant is choosing between organic and inorganic coagulants.
What is the purpose of coagulation?
The primary purpose of the coagulation for water treatment, or the flocculation process, is the removal of turbidity from the water. Turbidity is a cloudy appearance of water caused by small particles suspended therein. Water with little or no turbidity will be clear.
What are the three processes of coagulation?
As I mentioned above, the chemistry of coagulation/flocculation consists of three processes – flash mix, coagulation, and flocculation. Each of these processes is briefly explained below.
What is the end product of a well-regulated coagulation/flocculation process?
Floc : The end product of a well-regulated coagulation/flocculation process is water in which the majority of the turbidity has been collected into floc, clumps of bacteria and particulate impurities that have come together and formed a cluster. The floc will then settle out in the sedimentation basin, with remaining floc being removed in the filter.
What happens when water flows over the ground?
As surface water flows over the ground to streams, through streams, and then through rivers, the water picks up a large quantity of particles. As a result, while aeration is more commonly required for groundwater, treatment involving coagulation and flocculation is typical of surface water.
What is the maximum turbidity in water?
Water with a high turbidity can be very difficult or impossible to properly disinfect. As a result, the maximum allowable level of turbidity in water is 0.5 NTU, while the recommended level is about 0.1 NTU. ( NTU, or TU, stands for nephelometric turbidity units, a measurement of the turbidity of water.)
Why is coagulation important in wastewater treatment?
Today, coagulation and flocculation are still essential components of treatment processes, e.g. for reducing water turbidity. Wastewater treatment operations also require coagulation for chemical phosphorus removal and for reducing suspended solids.
What is coagulation in water?
Through coagulation, industrial water supplies are put into the perfect chemical state for easy mechanical filtration. Once the flocs settle at the bottom of your clarifier, equipment like a filter press can then take those larger clumps of aggregated particles and remove them, delivering clean water back into your system.
What is the name of the cationic coagulant that neutralizes the negative charge of colloids in water?
PolyAMINEs and PolyDADMACs – These cationic coagulants work by charge neutralization alone and are the most widely used organic coagulants. PolyAMINEs and PolyDADMACs neutralize the negative charge of colloids in your water, forming a spongy mass called a “microfloc.” Since they only coagulate through charge neutralization, they don’t offer any advantages in regard to the sweep-floc mechanism (explained later with inorganic coagulants).
What is the purpose of coagulant?
The primary purpose of using a coagulant besides removing vary fine particles from suspension is that this process results also in less turbidity of the water, i.e. clearer water.
What happens when coagulants are positive?
With coagulants’ positive charge, the negatively charged particles in the water are neutralized. This causes the suspended solids in the water to bind together into larger flocs. These larger flocs begin to settle at the base of the water supply. The larger the size of the particles, the quicker the floc settles.
What is the process of adding coagulants to water?
These help to clean the water by absorbing impurities in the water as they fall. This process is known as the “sweep-floc” mechanism. However, this can add to the overall sludge volume that must be treated and removed, so it’s not the right choice in every scenario.
When was coagulation used in water treatment?
Coagulation in Wastewater Treatment has been used to clarify water since ancient times – as early as 2000BC, when the Egyptians used almonds to clarify river water. There is also evidence to suggest that the Romans were using alum as a coagulant at around 77AD.
