:strip_exif(true):strip_icc(true):no_upscale(true):quality(65)/d1vhqlrjc8h82r.cloudfront.net/08-18-2021/t_147f523aa72848d19821e59e9ad64d9c_name_image.jpg)
- Results from a phase 3 clinical trial show that Regeneron’s antibody cocktail has the ability to cut the risk of COVID-19 hospitalization and death by 70 percent.
- The treatment also shortened the duration of COVID-19 symptoms by 4 days.
- The two antibodies work similarly to the antibodies the immune system naturally produces to fight the coronavirus.
Full Answer
What drugs are in Regeneron?
The drugs— Regeneron Pharmaceuticals Inc.’s Regen-Cov and Eli Lilly & Co.’s bamlanivimab and etesevimab—shouldn’t be used in any U.S. states, territories or jurisdictions at this time, the Food and Drug Administration said Monday.
Is Regeneron better than remdesivir?
Remdesivir: Remdesivir is an ... with remdesivir had a recovery time one day shorter and a 30% chance of improved clinical status after 15 days than did ... Regeneron released more data in October ...
Who is eligible for Regeneron treatment?
The company said REGN-COV2, a combination of two synthetic antibodies that bind to the SARS-CoV-2 virus, should be used in infected people at high risk of progressing to hospitalization, such as those with obesity or heart disease.
How often can you get Regeneron?
There is a 10-day window to get the treatment after symptom onset, according to the Centers for Disease Control and Prevention. If you wait longer, “by then the virus has ravaged the body. And there’s not a whole lot the infusion of monoclonal antibodies is going to do to be able to reverse the course of the disease,” Fuller said.
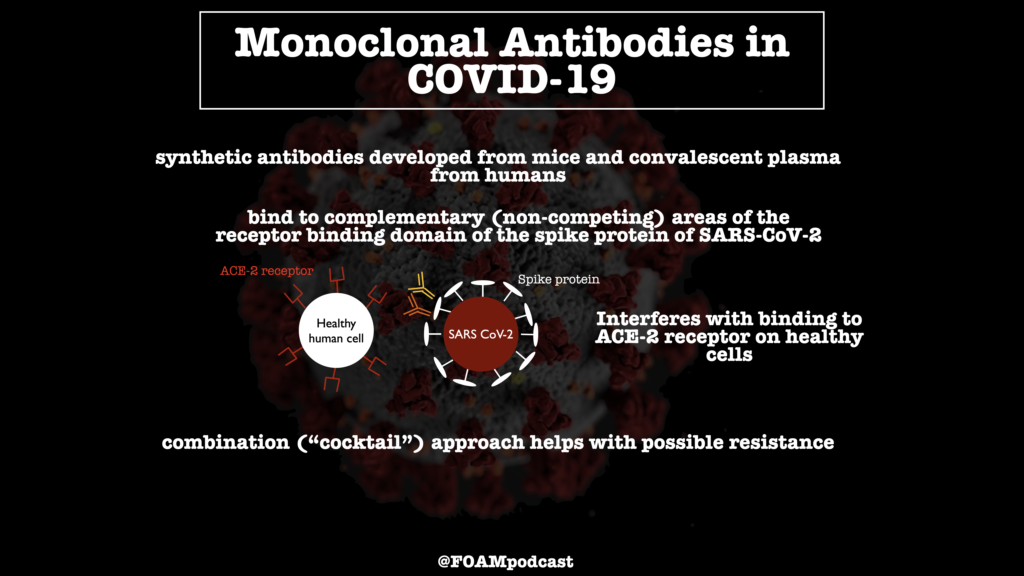
How do monoclonal antibodies work against COVID-19?
Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.
How often can you take Paxlovid?
“With Paxlovid, you take three pills, twice a day, for a total of five days," says Rachel Kenney, a pharmacist at Henry Ford Health. "It helps your body fight off the virus, preventing it from replicating before it becomes serious.”
How long do COVID-19 antibodies last?
At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity.
Does Paxlovid cause diarrhea?
“Paxlovid is usually very well-tolerated,” he says. Common side effects, which are usually mild, include: Altered or impaired sense of taste. Diarrhea.
Does Paxlovid have side-effects?
“Paxlovid is usually very well-tolerated,” he says. Common side effects, which are usually mild, include: Altered or impaired sense of taste. Diarrhea.
What is Paxlovid for COVID-19?
Paxlovid (nirmatrelvir tablets; ritonavir tablets) is a prescription oral antiviral drug that reduces the risk of hospitalization and death for patients with mild-to-moderate COVID-19 who are at risk of disease progression and severe illness (1).
Can you get COVID-19 if you already had it and have antibodies?
It is important to remember that some people with antibodies to SARS-CoV-2 may become infected after vaccination (vaccine breakthrough infection) or after recovering from a past infection (reinfected).
How long does it take for immunity to wane after receiving the COVID-19 vaccine?
A study published by the U.S. Centers for Disease Control and Prevention found that immunity against severe COVID-19 begins to wane four months after receiving a so-called "booster" third dose of the Pfizer or Moderna vaccines.
Can I get reinfected with COVID-19?
Studies suggest that reinfection with SARS-CoV-2 with the same virus variant as the initial infection or reinfection with a different variant are both possible; early reinfection within 90 days of the initial infection can occur.
Can COVID-19 cause diarrhea?
COVID-19 mainly attacks the cells lining your airways. This makes it hard for you to breathe and can lead to pneumonia. But researchers think the illness also may harm your digestive tract and liver tissue.
What are some of the symptoms of the Omicron COVID-19 variant?
All of the variants, including delta and omicron, cause similar COVID-19 symptoms, including cough, fever and fatigue. There is some evidence that fewer people with omicron lose their taste and smell.
Does COVID-19 cause digestive symptoms?
If you have diarrhea, nausea, or vomiting, it doesn't mean that you have COVID-19. But it's wise to pay extra attention to your symptoms during this pandemic, especially if you have a health condition that raises your chances for an infection or if you live in an area where the new coronavirus is widespread.
What is Regeneron's process?
Regeneron’s process starts with our deep belief in the power of genetics and our fundamental understanding of basic biology. In the early 2000s, we generated one of the first “knockout” mice in biotech history, which allowed us to study how certain genes (or the absence of certain genes) affect health and disease.
What is an antibody medicine?
Antibodies as medicines. Antibody medicines are based on key principles of biology and mimic the natural defenses and pathways of the human body and immune system. Scientists look at how antibodies work in order to replicate and optimize them against specific disease targets. Now antibody medicines can be created in a lab ...
Why is antibody testing necessary?
In order to test the medicine, it’s necessary to find a way to increase production of the antibody in small but consistent batches.
What are the diseases that antibody drugs have been proven to treat?
Antibody medicines have been proven to change lives and have altered the course of the treatment of serious diseases like asthma, cancer, heart disease, rheumatoid arthritis and severe eczema over the past several decades. MORE ON ANTIBODY SCIENCE.
What is bispecific antibody?
Bispecific antibodies are engineered to bind two different molecular targets – meaning each arm of the “Y” reaches out to attach to something different. For example, one arm may attach to a tumor cell while the other captures a T-cell.
Why do antibodies have to be manufactured?
Antibody medicines must be manufactured on a large scale in order for them to be available for appropriate patients. This is a highly integrated bioengineering process that requires consistency and reliability to meet regulatory requirements. At Regeneron, we don’t believe that all antibody medicines are created equal.
How many antigen binding sites are there in each arm of the Y?
Each antibody has two antigen-binding sites (one on each arm of the Y) and works to neutralize these invaders. Antibody medicines are designed to emulate this natural disease-fighting process and, with the help of scientific innovation, do much, much more.
Can you make antibodies from convalescent plasma?
But it is not possible to make convalescent plasma a mass treatment.
Is Regeneron a late stage drug?
Regeneron is also running late-stage trials for hospitalized COVID-19 patients in the UK and for the drug's potential use to prevent household contacts of COVID-19 patients from being infected.
What is Regeneron?
Regeneron is a monoclonal antibody treatment, a combination of two antibodies called casirivimab and imdevimab, that has been shown, in recent clinical trials, to reduce COVID-19 related hospitalization or deaths in high-risk patients by approximately 70%.
How Does Regeneron Work?
Regeneron consists of monoclonal antibodies. Monoclonal antibodies are like the antibodies that your body makes to fight against infections, however, they are made in the labs of pharmaceutical companies, like Regeneron. These antibodies are specially designed to target the coronavirus spike protein.
What if I Am Already Sick?
If you are already infected with COVID-19, monoclonal antibodies can prevent the development of severe symptoms that require hospitalization.
When Should You Receive a Regeneron Infusion?
Timing is a critical component of Regeneron treatment. The earlier you receive Regeneron, the more effective it is at treating or preventing COVID-19. Studies have shown that Regeneron is most effective within the first 4 to 5 days of symptoms. Monoclonal antibodies cannot be given after 10 days of symptoms.
How is Regeneron Different From The COVID-19 Vaccine?
The purpose of a vaccine is to help stimulate and prepare your immune system to respond if or when you are exposed to the virus. A vaccine prepares your immune system to create all these antibodies before they are needed.
What Are The Possible Side Effects of Regeneron?
Allergic reactions can happen during and after infusion of Regeneron. The symptoms of an allergic reaction can consists of but are not limited to the following:
How Long Does A Regeneron Infusion Take?
If you are receiving a Regeneron infusion, the infusion will take approximately 20 to 50 minutes or longer.
What is the NICA for Regeneron?
National Infusion Center. Association (NICA) Additional REGEN-COV resources for patients and providers. Regeneron is collaborating with Roche to increase global supply of REGEN-COV, with expected production of at least 2 million treatment doses per year, beginning in 2021.
Is Regen-COV approved for use in hospital?
Limitations of Authorized Use. REGEN-COV is not authorized for use in patients who are hospitalized due to COVID-19, who require oxygen therapy due to COVID-19, or who require an increase in baseline oxygen flow rate due to COVID-19 in those on chronic oxygen therapy due to underlying non-COVID-19 related comorbidity.
Is Regen-COV a comorbidity?
Therefore, REGEN-COV is not authorized for use in patients who are hospitalized due to COVID-19, OR who require oxygen therapy due to COVID-19, OR who require an increase in baseline oxygen flow rate due to COVID-19 in those on chronic oxygen therapy due to underlying non-COVID-19–related comorbidity. Adverse Reactions:
Does Regen-CoV have potency?
Similarly, the FDA authorized factsheet for Health Care Providers affirms that REGEN-COV retains potency against the variants first identified in the UK, South Africa, Brazil, California, New York or India, which are now classified by the WHO as Alpha, Beta, Gamma, Iota, Epsilon and Delta respectively.
Is REGEN-COV a monoclonal antibody?
Monoclonal antibodies, such as REGEN-COV, may be associated with worse clinical outcomes when administered to hospitalized patients with COVID-19 requiring high-flow oxygen or mechanical ventilation. Please see below for Important Safety Information.
Can you have two complementary antibodies in one?
With two complementary antibodies in one therapeutic, even if one antibody has reduced potency in response to a variant strain of the virus, the risk of the combination losing efficacy is diminished, as the virus would need to mutate in multiple distinct locations to evade both antibodies.
What exactly is in a monoclonal antibody treatment and how do they work?
In the United States, there are three monoclonal antibody treatments with FDA emergency use authorization for the treatment of COVID-19: bamlanivimab plus etesevimab, developed by Eli Lilly; casirivimab plus imdevimab, made by Regeneron Pharmaceuticals; and sotrovimab, which is manufactured by GlaxoSmithKline.
Who is eligible for monoclonal antibody treatment?
If you believe you are at high risk for progression of severe COVID-19, including hospitalization or death, you may be eligible for the the COVID-19 antibody cocktails.
How effective is it?
Ginde said it can be a life-saving treatment when administered in time. Numerous trials have shown that the treatment can be effective at reducing the risk of hospitalization and death for people at risk of severe COVID.
When do I need to get the treatment in order for it to work?
The monoclonal antibody treatments are meant for mild to moderate COVID cases in adults and children over 12 to prevent the progression of severe COVID.
How can I get a monoclonal antibody treatment for COVID-19?
The ease of access varies state by state, as the Department of Health and Human Services determines how much of the national supply gets distributed on a weekly basis. Then, different state and territorial health departments decide which areas receive it and how much.
Are there side effects?
It’s rare but possible to have side effects. At least 1% of subjects receiving Regeneron’s antibody cocktail in a Phase 3 trial got skin redness and itchiness at the injection site, according to the FDA.
How much does it cost?
The federal government is covering the cost of the monoclonal antibody therapies, so it is free to get, but there might be an administration cost billed to your insurance if you have one.
