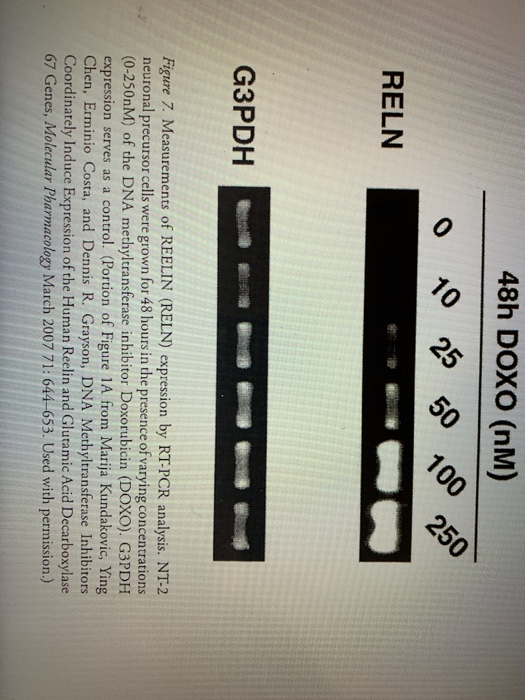
In reference to Figure 3, what is the effect of doxorubicin treatment on the expression of reelin in NT-2 cells? How do increasing amounts of doxorubicin affect reelin expression in these cells? The drug doxorubicin affects DNA methylation via inhibiting DNA methyl transferase 1 enzyme.
How does doxorubicin affect reelin expression?
The doxorubicin decreased levels of DNMT1 were previously reported exploited to actively repress the GAD67 and reelin promoter eventually significantly increasing expression of reelin and GAD67 (43).
How does a drug like doxorubicin affect DNA methylation?
Herein, we show that the enzymatic activity of DNMT1, the primary DNA methyltransferase in mammalian cells, is inhibited by DNA intercalators, such as doxorubicin, in an in vitro assay. Enzymatic analyses indicate that doxorubicin inhibits the catalytic activity of DNMT1 via DNA intercalation.
What is the role of Reelin in the brain?
During embryonic development and adulthood, Reelin exerts several important functions in the brain including the regulation of neuronal migration, dendritic growth and branching, dendritic spine formation, synaptogenesis and synaptic plasticity.
How can you inhibit DNA methylation?
The most well characterized and widely used drugs to inhibit DNA cytosine methylation and reactivate silenced genes are several nucleoside analogs, including 5-azacytidine (5-Aza-CR) and 5-aza-2′-deoxycytidine (5-Aza-CdR) (12,13), and several non-nucleoside drugs, including procainamide (14,15).
What kind of drug is doxorubicin?
Doxorubicin is a type of chemotherapy drug called an anthracycline. It slows or stops the growth of cancer cells by blocking an enzyme called topo isomerase 2. Cancer cells need this enzyme to divide and grow. You might have doxorubicin in combination with other chemotherapy drugs.
What is reelin expression?
In the adult brain, reelin is expressed by GABA-ergic interneurons of the cortex and glutamatergic cerebellar neurons, the glutamatergic stellate cells and fan cells in the superficial entorhinal cortex that are supposed to carry a role in encoding new episodic memories, and by the few extant Cajal-Retzius cells.
What would be the effect of inhibiting the effects of the protein reelin during development?
Absence of Reelin during development leads to abnormal corticogenesis, Purkinje cell loss and ataxia. Reductions in levels of Reelin during adult life may cause cognitive deficits, as seen in autism, schizophrenia, bipolar disorder and lissencephaly.
What is the role of reelin in the brain before and after birth?
After birth, reelin likely plays a role in many brain processes, including the extension of axons and dendrites, which are specialized outgrowths from nerve cells that are essential for the transmission of nerve impulses.
What is the role of Reelin in cancer?
Recently, reelin was found to be epigenetically silenced in gastric and pancreatic cancers in which down-regulation was associated with increased migratory ability and reduced survival . Here we analyzed reelin expression by immunohistochemistry in 17 normal breast tissue samples from reduction mammoplasties and in two independent tissue microarrays of 136 and more than 2000 breast cancer biopsy samples, respectively. Results were analyzed with regard to clinical parameters, including BRE (Bloom, Richardson, Elston) grade, nodal status, estrogen receptor and HER2 status, and overall survival. Reelin was expressed in the luminal epithelium and myoepithelium of the normal human breast but not in cancerous breasts. Loss of reelin protein expression correlated significantly with decreased survival ( P = 0.01) and positive lymph node status ( P < 0.001). By measuring reelin expression and promoter methylation status in 39 primary breast tumors, as well as in breast cancer-derived cell lines before and after decitabine treatment, we established that reelin expression levels correlated inversely with promoter methylation status, whereas demethylation increased reelin mRNA expression in vitro. Reelin overexpression in MDA-MB231 cells, as well as incubation with recombinant reelin, suppressed cell migration, invadopodia formation, and invasiveness in vitro. We conclude that reelin may play an important role in controlling invasiveness and metastatic potential of breast cancer cells and that its expression is controlled by promoter methylation.
What was used in the Ludwig trial?
The material used in the pilot study was taken from a cohort of patients who were enrolled into the Ludwig Trial V Ludwig Breast Cancer Study Group. 25 The cancers used were representative of the trial cohort. From 1981 to 1985, the International Breast Cancer Study Group (formerly the Ludwig Breast Cancer Study Group) conducted a randomized clinical study to assess the effect of early commencement of adjuvant cyclophosphamide, methotrexate, and 5-fluorouracil chemotherapy in patients with lymph node-negative and lymph node-positive breast carcinoma.
What is the MDA-MB231 vector?
MDA-MB231 cells were transfected with a reelin expression vector (pCrl; generous gift from T. Curran, The Children's Hospital of Philadelphia, Philadelphia, PA). This vector expresses full-length reelin under control of a cytomegalovirus promoter in a pcDNA3 backbone (neomycin-resistant) and was first cloned by D'Arcangelo et al. 23 Cells were transfected at 80% confluence with 2 μg of unlinearized plasmid DNA, using Lipofectamine 2000 (Invitrogen) for 24 hours. Stable transfectants were selected by growing cells in 500 μg/ml G418. After serial dilution in a 96-well plate, 10 clones were cultured, and reelin protein levels were analyzed by Western blotting (data not shown). Finally, one clone expressing the full-length reelin and one clone expressing a truncated fragment of reelin were selected for further investigation. The procedure was repeated using linearized pCrl and two further clones were selected that showed either full-length or truncated reelin expression.
Does reelin affect tumor spread?
It is therefore possible that reelin negatively affects tumor invasiveness. Invadopodia are invasion-associated protrusions that have been suggested to facilitate cancer cell-induced degradation of the extracellular matrix and subsequent invasion and can therefore be used as a measure for the invasive potential of a cell. Invadopodia can be visualized when cells are grown on a fluorescent Oregon Green 488 gelatin matrix as areas of gelatin degradation (dark spots) with punctate actin staining. The invadopodia formation ability of reelin-overexpressing MDA-MB231 cells was therefore compared with that of the highly invasive parental cell line and with that of MDA-MB231 cells expressing the C-terminally truncated form of reelin. Cells were grown on fluorescent gelatin for 5 hours, and invadopodia formation was assessed by counting the total number of invadopodia and of invadopodia-forming cells. Cells that overexpressed full-length reelin showed a strongly reduced number of invadopodia and a rounded appearance ( Figure 5, A, middle row ), whereas those cells expressing the truncated reelin (bottom row) showed a level of invadopodia formation similar to that of the parental cell line ( Figure 5A, top row; B and C ). In addition, full-length reelin-expressing MDA-MB231 cells showed increased actin stress fiber formation (phalloidin staining) instead of the punctate actin pattern associated with invadopodia, which could be seen in the other two cell lines. This result was reproducible using independently selected reelin-expressing MDA-MB231 stable transfectants (data not shown). A similar, although weaker, effect was observed when the parental MDA-MB231 cell line was incubated with reelin collected from reelin-expressing HEK293 cells. In two separate experiments (gray and black bars) a stronger reduction in gelatin degradation and in the number of gelatin-degrading cells was observed in the presence of reelin-containing medium than with control medium from green fluorescent protein-overexpressing HEK293 cells (Figure 5, D and E).
Is Reelin a biological function?
Until recently, a biological function for reelin had only been found in the developing and adult brain, although its expression has been described in many other organs. 17, 18 The idea that reelin could indeed function outside the brain was supported by recent studies, 19, 20, 21 which linked the reelin signaling pathway to prostate, gastric, and pancreatic cancer, respectively. These studies showed that local reelin expression is an important prognostic factor in these systems, albeit in opposite directions. Whereas reelin down-regulation was found in pancreatic cancer and gastric cancer, in prostate cancer reelin expression was associated with high-grade disease. Using two independent cohorts of breast cancer patients, we have now shown for the first time that reelin protein is expressed in the human breast epithelium and that loss of expression is associated with poor prognosis in breast cancer. Although one study did not reach statistical significance because of the low number of patients, our study of more than 2000 tissue cores clearly showed a statistically significant link between loss of strong reelin immunoreactivity and poor survival. Loss of reelin expression was also associated with positive lymph node status, suggesting that it may play a role in suppressing metastasis formation. This finding is in agreement with the findings by Sato et al 20 and Dohi et al 21 that epigenetic silencing of reelin is associated with pancreatic and gastric cancer. Sato et al suggested that loss of reelin expression is accompanied by an increase in migratory and invasive ability, as shown in an in vitro invasion assay. We have now been able to confirm these results in breast cancer. In the developing cerebral cortex, it is thought that one of the functions of reelin may be to provide a stop signal in a paracrine fashion for migrating neurons to inhibit these cells from entering the marginal zone. 33, 34, 35 Reeler mice and those with a defect in the receptor VLDLR both showed increased invasion of the marginal zone by migrating neurons. It is conceivable that in the breast epithelium reelin could act in an autocrine fashion to suppress cell migration/invasion. However, it is not clear whether reelin signals in the breast through the same pathways as those that have been described in the brain. Indeed, Dulabon et al 34 showed that reelin can inhibit cortical neuron migration through modulation of integrin-dependent cell adhesion, in particular integrins α 3 β 1, which have also been associated with breast cancer metastasis formation. 36 It is therefore feasible that reelin may reduce cell migration through modulation of integrin-matrix interaction. This hypothesis is currently under investigation.
Does methylation affect mRNA?
Most likely, this methylation will interfere with the assembly of regulatory proteins, such as transcription factors, as the enhancer, and RNA polymerase II near the transcription start site. Increased methylation at these sites will likely reduce reelin mRNA expression
Does doxorubicin help with schizophrenia?
Using doxorubicin to inhibit DNA methyltransferase would relieve the hypermethylation of the promoter, leading to increased reelin expression and could potentially treat schizophrenia
