- Testing Pregnant Women. Most babies born to women who tested positive for GBS bacteria do not need treatment if their mother received antibiotics during labor.
- Antibiotics during Labor. Doctors give antibiotics to women who are at increased risk of having a baby who will develop GBS disease.
- Vaccination. Currently, there is no vaccine to help pregnant women protect their newborns from GBS bacteria and disease.
- Strategies Proven Not to Work
What is the treatment for sepsis in newborns?
Medical Care. A neonate with sepsis may require treatment aimed at the overwhelming systemic effects of the disease. Cardiopulmonary support and intravenous (IV) nutrition may be required during the acute phase of the illness until the infant’s condition stabilizes.
Can GBS cause sepsis in babies?
GBS infection can lead to meningitis, pneumonia, or sepsis. Meningitis is more common in a baby who has a GBS infection happen a week to several months after birth. What can I do to prevent group B strep in my child?
Can antibiotics prevent GBS disease in newborns?
There is a rare chance (about 1 in 10,000 women) of having a severe allergic reaction that requires emergency treatment. Antibiotics are very effective at preventing GBS disease in newborns. Consider the following examples: Emma’s baby is 20 times more likely go get GBS disease compared to Tanya’s baby.
What is the treatment for Guillain-Barré syndrome (GBS) during pregnancy?
It has been demonstrated that the administration of intravenous penicillin at least 4 h before delivery to mothers colonized with GBS is highly effective in preventing perinatal transmission and early-onset invasive infection in the newborn (8).

How is the GBS infected baby treated?
If your baby has a GBS infection, how is he treated? It's important to try and prevent a newborn from getting GBS. But if a baby does get infected with early-onset GBS or late-onset GBS, he is treated with antibiotics through an IV.
What is the drug of choice for the treatment of neonatal sepsis?
The most commonly recommended and used first-line treatment for both early and late onset neonatal sepsis is a beta-lactam antibiotic (most commonly ampicillin, flucloxacillin and penicillin) combined with an aminoglycoside (most commonly gentamicin) [21, 31, 48, 51, 54,55,56,57].
What is the most important treatment of neonatal sepsis?
In late-onset hospital-acquired sepsis, initial therapy should include vancomycin (active against methicillin-resistant S. aureus; see table Vancomycin Dosage for Neonates ) plus an aminoglycoside.
What is adequate treatment for GBS?
Adequate prophylaxis involves antibiotic treatment for at least 4 hours prior to delivery. If given for less than 4 hours, this is considered inadequate prophylaxis. Antibiotics proven to be effective include: Penicillin (5 million units initial IV dose, then 2.5-3 million units q4 hours until delivery)
Which antibiotics is considered safe to use in neonates?
The World Health Organization (WHO) recommends paren- teral antibiotic therapy (eg, benzylpenicillin or ampicillin plus an aminoglycoside such as gentamicin) in a health facility as the standard treatment for serious neonatal infections (ie, septicemia, pneumonia, and meningitis) in developing countries.
How will you manage a case of neonatal sepsis?
Immediate empirical administration of broad-spectrum anti-microbials, aggressive fluid resuscitation, and vaso-active or inotropic support (or both) are the mainstays of the therapeutic management of neonatal sepsis.
How long does it take to treat neonatal sepsis?
The duration of empirical antibiotic therapy in neonates should be 48–72 hours pending culture results for suspected sepsis. Until further evidence, the current recommendation of 10–14 days of antimicrobial treatment is appropriate for blood-culture-positive sepsis without meningitis.
How is neonatal infection treated?
In the United States and Canada, the current approach to the treatment of early-onset neonatal sepsis includes the administration of combined intravenous (IV) aminoglycoside and expanded-spectrum penicillin antibiotic therapy.
Does ampicillin treat sepsis?
Ampicillin and gentamicin are usually effective against all the bacterial agents causing community-acquired sepsis in neonates as this combination has traditionally been considered to have activity against both Gram-positive and Gram-negative organisms in the neonatal period (22).
What antibiotics are given for GBS?
Doctors usually treat GBS disease with a type of antibiotic called beta-lactams, which includes penicillin and ampicillin. Sometimes people with soft tissue and bone infections may need additional treatment, such as surgery.
What medications are administered to treat streptococcus B hemolytic infection during labor?
Intravenous penicillin G is the treatment of choice for intrapartum antibiotic prophylaxis against Group B Streptococcus[1][11][1]. Penicillin G 5 million units intravenous is administered as a loading dose, followed by 2.5 to 3 million units every 4 hours during labor until delivery[1].
Is vancomycin considered adequate treatment for GBS?
Vancomycin is recommend for penicillin-allergic patients at high risk for anaphylaxis whose susceptibility to both agents is unknown or whose antimicrobial susceptibility testing results show the GBS isolate to be intrinsically resistant to clindamycin or positive for inducible clindamycin resistance.
What is the procedure to test for sepsis in newborns?
Tests for sepsis in newborns can include: Spinal tap (also known as lumbar puncture) to test for meningitis. A spinal tap is a procedure in which a very small needle is inserted into the space around your child’s spine to withdraw spinal fluid to test for infections.
How early can a baby get sepsis?
Newborns can get sepsis in several different ways: If the mother has an infection of the amniotic fluid (a condition known as chorioamnionitis) If the mother’s water breaks early (more than 18 hours before the baby is born) If the baby is being treated for another condition while still in the hospital.
What is the risk factor for sepsis?
Low birth weight of the infant (risk factor for sepsis) If the mother’s water breaks early (more than 18 hours before the baby is born) If the baby is being treated for another condition while still in the hospital. If the mother’s birth canal is colonized with bacteria.
What causes sepsis in the body?
Bacterial infections are the most common cause of sepsis. However, sepsis can also be caused by fungi, parasites or viruses. The infection can be located in any of a number of places throughout the body.
How does intrapartum antibiotics prevent GBS?
30,–32,63 IAP is hypothesized to prevent neonatal GBS disease in 3 ways: (1) by temporarily decreasing maternal vaginal GBS colonization burden; (2) by preventing surface and mucus membrane colonization of the fetus or newborn; and (3) by reaching levels in newborn bloodstream above the minimum inhibitory concentration (MIC) of the antibiotic for killing group B streptococci. 30,31 Current clinical practices are focused on the identification of women at highest risk of GBS colonization and/or of transmission of group B streptococci to the newborn infant to facilitate targeted administration of IAP.
How long does it take for a newborn to get a GBS EOD?
GBS EOD primarily presents clinically at or shortly after birth. The majority of infants become symptomatic by 12 to 24 hours of age 11,–13; during the 2006–2015 period of ABCs, 94.7% of GBS EOD cases were diagnosed ≤48 hours after birth. 6 The incidence of newborn infants discharged from a birth hospital and readmitted to a hospital with GBS EOD within 6 days of age was approximately 0.3 cases per 100 000 live births in the ABCs. Although potentially an underestimate, this finding was consistent with data from the Northern California Kaiser Permanente health care system, which found the incidence of newborn infants readmitted to the hospital within the first week after hospital discharge with culture-confirmed infection attributable to any bacteria to be approximately 5 cases per 100 000 live births. 14 Globally, outside the United States, an estimated 200 000 cases of GBS EOD occurred in 2015. Stillbirth, GBS EOD, and late-onset GBS cases combined contribute to an estimated 150 000 fetal and neonatal deaths throughout the world, with the largest concentration of GBS perinatal deaths occurring in Africa. 15
What is a GBS EOD?
GBS EOD is defined as isolation of group B Streptococcus organisms from blood, cerebrospinal fluid (CSF), or another normally sterile site from birth through 6 days of age. 8 Active Bacterial Core surveillance (ABCs), performed in collaboration with the CDC in 10 states from 2006 to 2015, found that the overall incidence of GBS EOD declined from 0.37 cases per 1000 live births in 2006 to 0.23 cases per 1000 live births in 2015. 6 Meningitis was diagnosed in 9.5% of infants with GBS EOD. CSF culture-positive GBS EOD occurred in the absence of bacteremia in 9.1% of early-onset meningitis cases (incidence: approximately 2.5 cases per 1 million live births). 6 Infants born at <37 weeks’ gestation account for 28% of all GBS cases; approximately 15% of cases occur among preterm infants with very low birth weight (<1500 g). 6,9 Overall, GBS infection accounts for approximately 45% of all cases of culture-confirmed EOS among term infants and approximately 25% of all EOS cases that occur among infants with very low birth weight. 9,10 Death attributable to GBS EOD occurs primarily among preterm infants: the current case fatality ratio is 2.1% among term infants and 19.2% among those born at <37 weeks’ gestation. 6
Why do pregnant women receive antibiotics?
GBS IAP and the administration of intrapartum antibiotics because of concern for maternal intraamniotic infection combined result in approximately 30% of pregnant women receiving antibiotics around the time of delivery. The composition of the gut microbiota develops and diversifies from birth through early childhood, and disruption of the microbiota during this critical period may have enduring health consequences. Perinatal antibiotic administration is associated with abnormal host development in animal models. 106,–108 Human epidemiological studies have associated early infant antibiotic exposures with increased risks of atopic and allergic disorders as well as increases in early childhood weight gain. 109,–112 Several human studies report differences in the composition of the maternal vaginal 113 and infant gut microbiome associated with the use of IAP, with effects on both the diversity and richness of identified species. 114,–119 The differences observed vary with the mode of delivery and duration of breastfeeding; differences were detected as long as 12 months after birth in 1 study. 115 Changes in the initial constitution of neonatal gut microbiota in response to IAP are not unexpected; GBS IAP is administered with the intent of altering the content of the newborn infant’s initial microflora exposure at delivery to decrease colonization with a significant neonatal pathogen. Whether the secondary effects of IAP on the microbiome influence short- and long-term childhood health outcomes is unknown and an area of active investigation.
What are the symptoms of GBS EOD?
Newborn infants with GBS EOD may present with signs of illness ranging from tachycardia, tachypnea, or lethargy to severe cardiorespiratory failure, persistent pulmonary hypertension of the newborn, and perinatal encephalopathy. GBS LOD most commonly occurs as bacteremia without a focus and is often characterized by fever (≥38°C) as well as lethargy, poor feeding, irritability, tachypnea, grunting, or apnea. Infants with LOD meningitis can also have nonspecific signs such as irritability, poor feeding, vomiting, and temperature instability as well as signs suggestive of central nervous systems involvement such as bulging fontanelle or seizures. Focal syndromes such as pneumonia, bone or joint infection, cellulitis, or adenitis often have findings that point to the site of infection. Osteomyelitis frequently is insidious and may not be associated with fever. Complications are common for meningitis and can include neurodevelopment impairment, hearing loss, persistent seizure disorders, or cerebrovascular disease. 98
What is the IAP for GBS?
Updated ACOG recommendations address the indications for IAP. 7 IAP at the time of presentation for delivery is indicated for all women with GBS colonization identified by antenatal vaginal-rectal culture, for women with GBS bacteriuria identified at any point during pregnancy, for women with a history of a previous infant with GBS disease, and for women who present in preterm labor and/or with PROM at <37 0/7 weeks’ gestation. Women who present in labor at ≥37 0/7 weeks’ gestation with unknown GBS status should receive IAP if risk factors develop during labor (maternal intrapartum temperature ≥100.4°F [38°C] or duration of ROM ≥18 hours) or if the result of an available point-of-care NAAT is positive for group B streptococci. If a woman with unknown status has a negative point-of-care NAAT test result but develops intrapartum risk factors, IAP should be administered because the sensitivity of the NAAT may be decreased without an enrichment incubation step. Women with GBS colonization in one pregnancy have an estimated 50% risk of colonization in a subsequent pregnancy. 64 Therefore, current ACOG recommendations state that if a woman with unknown GBS status presents in labor and is known to have had GBS colonization in a previous pregnancy, IAP may be considered.
What is a late onset GBS?
GBS late-onset disease (LOD) is defined as isolation of GBS from a normally sterile site from 7 to 89 days of age. 8 Rarely, very–late-onset GBS disease may occur after 3 months of age, primarily among infants born very preterm or infants with immunodeficiency syndromes. 16,17 GBS LOD rates have not changed with widespread use of IAP. GBS LOD incidence was stable over the 2006–2015 ABCs study period, with an average incidence of 0.31 cases per 1000 live births. 6 The median age at presentation with GBS LOD was 34 days (interquartile range: 20–49 days). Bacteremia was identified in approximately 93% of GBS LOD, and bacteremia without focus was the most common form of disease. Group B streptococci were isolated from CSF in 20.7% of cases, and meningitis was diagnosed in 31.4% of cases. Cultures of bone and joint and peritoneal fluid yielded group B streptococci in 1.8% of cases. CSF culture-positive GBS LOD occurred in the absence of bacteremia in approximately 20% of late-onset meningitis cases (incidence: 1.9 cases per 100 000 live births). Infants born at <37 weeks’ gestation account for approximately 42% of all GBS LOD cases, and death attributable to GBS LOD occurs in preterm infants at roughly twice the rate of term infants (7.8% vs 3.4%, respectively). 6 GBS LOD complicated by meningitis has a higher patient fatality rate than those with other syndromes.
What to do if you test positive for GBS during pregnancy?
If you test positive for GBS during pregnancy, you will get intravenous (IV) antibiotics during labor. This lowers the risk that your baby will get the infection. Penicillin is the most common antibiotic given. Tell your healthcare provider if you have any medicine allergies.
What is the GBS infection?
Group B streptococcus (strep) is a type of bacteria. It can be found in the digestive tract, urinary tract, and genital area of adults. GBS infection usually does not cause problems in healthy women before pregnancy. But it can cause serious illness for a newborn baby. It may cause sepsis, pneumonia, meningitis, or seizures.
What is the most common cause of serious infections in newborns?
Group B strep is the most common cause of serious infections in newborns. GBS infection can lead to meningitis, pneumonia, or sepsis. Meningitis is more common in a baby who has a GBS infection happen a week to several months after birth.
How is group B strep diagnosed in a newborn?
Your baby's healthcare provider will test your baby’s body fluids, such as blood or spinal fluid.
How long does it take for a baby to show signs of B strep?
Newborn babies with group B strep usually have signs in the first 24 hours after birth . These signs may include: Babies who get group B strep a week or so after birth may have signs such as: Pregnant women may have group B strep without symptoms. When they have symptoms, these may include:
What are the symptoms of group B strep?
Having convulsions (seizure) Babies who get group B strep a week or so after birth may have signs such as: Decreased movement of an arm or leg. Pain with movement of an arm or leg. Breathing problems. Fever.
Can a baby get group B strep?
Newborns are more likely to get group B strep infection if the mother has: Preterm labor. Early breaking of water (rupture of membranes) A long time between rupture of membranes and birth. Internal fetal monitoring during labor. Fever. A past pregnancy with a baby who had group B strep.
What is the transition from immune tolerance to maternal antigens?
Such a transition is extremely dynamic, as is the pathophysiology of infant sepsis, which is dependent on many infant, maternal , and environmental factors.
Is sepsis still prevalent?
Introduction: Mortality due to sepsis is still prevalent, peaking at extreme ages of life including infancy. Despite many efforts, the peculiarity of the infant immune system has limited further advances in its treatment.
Does granulocyte transfusion affect sepsis?
They confirm how exogenous stimulation of the immune system through intravenous immunoglobulin, colony stimulating factors, and granulocyte transfusion have failed to impact on the prognosis of infant sepsis.
What is the treatment for neonatal sepsis?
In the United States and Canada, the current approach to the treatment of early-onset neonatal sepsis includes the administration of combined intravenous (IV) aminoglycoside and expanded-spectrum penicillin antibiotic therapy. This regimen provides coverage for gram-positive organisms, especially group B Streptococcus (GBS), ...
How to treat sepsis in neonates?
Historically, the treatment approach for suspected neonatal sepsis has included early aggressive initiation of antibiotics because of the neonate’s relative immunosuppression. Because early signs of sepsis in the newborn are nonspecific, diagnostic studies are often ordered and treatment initiated in neonates before the presence of sepsis has been proven. Moreover, because the American Academy of Pediatrics (AAP), [ 22, 23] the American College of Obstetricians and Gynecologists (ACOG), [ 24] and the Centers for Disease Control and Prevention (CDC) [ 25] all have recommended sepsis screening or treatment for various risk factors related to group B Streptococcus (GBS) infections, many asymptomatic neonates now undergo evaluation and are exposed to antibiotics.
What is the Kaiser Sepsis Calculator?
This model, commonly referred to as the “Kaiser Sepsis Calculator” has allowed for a significant reduction in the use of empiric antibiotics (from 5.0% of all births before implementation to 2.8% of all births thereafter) and obtaining blood cultures (12.8% of all births before implementation to < 5% of all births thereafter), without an increase in the rate of morbidity or mortality or readmissions for early-onset sepsis. [ 34, 35]
Why is IVIG used in neonatal sepsis?
The rationale for IVIg infusion is that it could provide type-specific antibodies, thereby improving opsonization and phagocytosis of bacterial organisms and enhancing complement activation and chemotaxis of neonatal neutrophils. It has been studied as a possible therapy for neonatal sepsis, but at present, the data do not support its routine use for this purpose. The main difficulties with IVIg therapy are as follows:
How long does it take for a sepsis test to be negative?
In most cases, 36-48 hours of negative culture results should allow the clinician to be confident that sepsis is absent; however, a small number of infants shown to have had sepsis by postmortem examination had negative culture results during their initial sepsis evaluation.
How long should a newborn be monitored for sepsis?
If neonatal sepsis was associated with meningitis, prolonged hypoxia, extracorporeal membrane oxygenation therapy, or brain abscess formation, the infant should be observed for several years to assess his/her neurodevelopment. If problems are found, the child should receive appropriate early intervention services and therapies.
How long does it take for a PCP to evaluate a newborn?
The primary care provider (PCP) should evaluate the infant with a history of neonatal sepsis within 1 week of discharge from the hospital. The infant can be assessed for superinfection and bacterial colonization associated with antibiotic therapy, especially if the therapy was prolonged.
What is neonatal sepsis?
Neonatal sepsis is a clinical syndrome characterized by systemic signs of infection and accompanied by bacteremia in the first month of life [1]. Sepsis occurring in the first 72 hours of life is defined as early-onset sepsis (EOS) [2] and that occurring beyond 72 hours as late-onset sepsis (LOS). Neonatal sepsis is associated with significant morbidity and mortality justifying prompt initiation of appropriate empirical antibiotic therapy. Knowledge of both the common pathogens causing septicemia in neonates and their antimicrobial susceptibility is essential in order to select appropriate antimicrobial treatment. Antimicrobial susceptibility patterns of pathogens vary geographically and are temporally dependent on local pathogens and patterns of antibiotic use. The aim of this paper is to review the literature regarding the appropriate choice and duration of antimicrobial therapy for suspected sepsis, culture-proven sepsis, and meningitis during neonatal period. We would like to focus mainly on the treatment of bacterial infection.
When should sepsis treatment begin?
Treatment of neonates with suspected sepsis or meningitis should commence as soon as appropriate cultures and intravenous access can be obtained. The initial choice of antimicrobial agents for empirical treatment is dependent on the knowledge of the probable pathogens based on the perinatal history, including any maternal symptoms, cultures, or instrumentation and susceptibility pattern of the organisms [18].
How long does it take for a sepsis culture to show up?
Although isolation of a pathogen in culture is a prerequisite for proven bacterial sepsis, culture results take at least 48–72 hours to be reported. The early and appropriate initiation of antimicrobial agents in high-risk neonates before the result of blood culture susceptibility is defined as “empirical antibiotic therapy.” Most infants admitted to the neonatal intensive care units (NICUs) receive empirical antibiotics when in fact the incidence of culture-proven EOS is only between 1 and 4.6 cases per 1000 live births [10, 11]. The clinical manifestations of neonatal sepsis are nonspecific, and the fear of missing the diagnosis is high because of the increased morbidity and mortality associated with sepsis. One study noted that the ratio of noninfected to blood-culture-positive neonates treated with antibiotics was between 15 : 1 and 28 : 1 [12]. Early and prompt empirical antibiotic therapy, based on risk-factor-driven decision in EOS [13, 14] and clinical symptoms in LOS [15], have been shown to reduce mortality in neonates. The risk factors for EOS include clinical chorioamnionitis, maternal intrapartum fever (>38.0°C), delivery at <37 weeks, rupture of membranes (ROM) >18 hours before delivery, maternal GBS colonization, previous infant with GBS infection, GBS bacteriuria, and inadequate intrapartum antibiotic prophylaxis [16]. Among infants who are discharged home, new onset of fever, cough, fast or difficult breathing, poor feeding, lethargy, and convulsions are indicators of sepsis and warrant initiation of appropriate antibiotic therapy [17].
What antibiotics are used for EOS?
Based on the common antibiotic susceptibilities of the predominant organism causing EOS, the recommended initial empiric therapy for a neonate with suspected bacterial sepsis and/or meningitis includes ampicillin and an aminoglycoside [22, 23]. This combination expands the antimicrobial spectrum and also offers synergistic bacterial killing. The other advantages are low cost and low rates of emergence of bacterial resistance [24].
How does sepsis affect neonatal health?
Neonatal sepsis is associated with increased mortality and morbidity including neurodevelopmental impairment and prolonged hospital stay. Signs and symptoms of sepsis are nonspecific, and empiric antimicrobial therapy is promptly initiated after obtaining appropriate cultures. However, many preterm and low birth weight infants who do not have infection receive antimicrobial agents during hospital stay. Prolonged and unnecessary use of antimicrobial agents is associated with deleterious effects on the host and the environment. Traditionally, the choice of antimicrobial agents is based on the local policy, and the duration of therapy is decided by the treating physician based on clinical symptoms and blood culture results. In this paper, we discuss briefly the causative organism of neonatal sepsis in both the developed and developing countries. We review the evidence for appropriate choice of empiric antimicrobial agents and optimal duration of therapy in neonates with suspected sepsis, culture-proven sepsis, and meningitis. Moreover, there is significant similarity between the causative organisms for early- and late-onset sepsis in developing countries. The choice of antibiotic described in this paper may be more applicable in developed countries.
What are the pathogens that cause neonatal sepsis?
In United States, the National Institute of Child Health and Development (NICHD) reported that the common pathogens causing EOS are group B Streptococcus (GBS) and Escherichia coli [2].
How long does it take for a meningitis to relapse after antibiotics?
Despite adequate antimicrobial therapy for 21 days or more , relapses may occur in meningitis caused by Gram-negative enteric bacilli [59].
What is the treatment for neonatal sepsis?
Treatment is initially with ampicillin plus either gentamicin or cefotaxime, narrowed to organism-specific drugs as soon as possible. (See also Sepsis and Septic Shock in adults and Overview of Neonatal Infections .) Neonatal sepsis occurs in 0.5 to 8.0/1000 births. The highest rates occur in.
How many infants develop invasive disease due to GBS?
Although only 1/100 of infants colonized develop invasive disease due to GBS, > 50% of those present within the first 6 hours of life. Nontypeable Haemophilus influenzae sepsis has also been identified in neonates, especially premature neonates.
How early can you tell if you have neonatal sepsis?
Particularly common early signs include. Fever is present in only 10 to 15% of neonates but, when sustained (eg, > 1 hour), generally indicates infection.
How early can neonatal sepsis start?
Onset of neonatal sepsis can be early (≤ 3 days of birth) or late (after 3 days).
Why is early diagnosis of neonatal sepsis important?
Early diagnosis of neonatal sepsis is important and requires awareness of risk factors (particular ly in LBW neonates) and a high index of suspicion when any neonate deviates from the norm in the first few weeks of life.
What causes early onset sepsis?
Most infants have symptoms within 6 hours of birth. Most cases are caused by group B streptococcus (GBS) and gram-negative enteric organisms ( predominantly Escherichia coli ). Vaginal or rectal cultures of women at term may show GBS colonization rates of up to 35%.
What is the most common cause of late onset sepsis?
Staphylococci account for 30 to 60% of late-onset cases and are most frequently due to intravascular devices (particularly central vascular catheters).
How many infants are asymptomatic at birth with GBS?
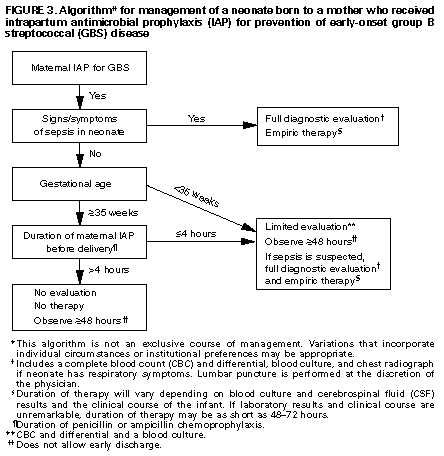
The risk of invasive early-onset GBS disease in an infant whose mother is GBS-positive and does not receive IAP is approximately 1% (21). Only one-quarter of these babies are asymptomatic at birth. This risk of significant disease probably does not justify routine empirical treatment in these circumstances, and careful observation with treatment at the first clinical sign of infection appears to be reasonable. Ninety-five per cent of infants with early-onset GBS infection present with clinical signs within 24 h (22) (either temperature instability, tachycardia, poor peripheral perfusion, respiratory distress or abnormal CBC). Four per cent of infected infants present between 24 h and 48 h of age, with only 1% developing signs after 48 h of age. Thus, prolonging hospitalization from 24 h to 48 h would require the observation of more than 2000 infants to detect each case of invasive infection. Therefore, if careful assessment of the infant at 24 h confirms that they remain well, discharge at that time may well be appropriate as long as adequate patient education and follow-up are ensured.
What is the most common organism that causes neonatal sepsis?
The most common single organism that causes early-onset neonatal sepsis is the group B streptococcus (GBS or Streptococcus agalactiae) (1). Invasive early-onset GBS disease has an incidence of approximately two per 1000 live-born infants in the absence of intrapartum antibiotic prophylaxis (IAP) (2,3), with a case-fatality rate of between 2% and 13% in recent studies (4–6). Therefore, preventive strategies have been promoted and recently endorsed by the Society of Obstetricians and Gynaecologists of Canada (7). It has been demonstrated that the administration of intravenous penicillin at least 4 h before delivery to mothers colonized with GBS is highly effective in preventing perinatal transmission and early-onset invasive infection in the newborn (8). The recommendations are to screen all mothers with rectovaginal cultures at 35 to 37 weeks, and treat those with positive cultures for GBS at the time they present in labour. This strategy leads to as many as 22% of all mothers in labour at term being treated with IAP to prevent disease in 0.2% of infants and prevent mortality in 0.01% of infants (9). In the United Kingdom, it was calculated that it would require 24,000 antepartum cultures and 7000 women in labour treated with antibiotics to prevent one neonatal death (10). As a consequence, other authorities have developed different recommendations, questioning whether routine IAP is an appropriate use of resources (10,11), and whether the pressure exerted for the development of bacterial resistance is justified. In Canada, the current incidence of invasive neonatal GBS disease is uncertain because there is no centralized or mandatory reporting system.
How long does it take for penicillin to kill GBS?
IAP with a penicillin for least 4 h is highly effective at eradicating GBS transmission (19), and thus preventing the majority of invasive neonatal GBS disease (evidence level 1b) (20). Therefore, if a GBS-positive woman receives intrapartum antibiotics for at least 4 h before delivery and if the newborn appears healthy and is more than 35 weeks gestational age, the newborn requires no therapy for prevention of early-onset GBS (recommendation grade A).
What are the signs of sepsis in infants?
The initial signs of sepsis may be subtle, and may include temperature instability, tachycardia, poor peripheral perfusion and respiratory distress. Because the progression of invasive disease is very rapid, any infant with clinical signs suggestive of infection should be treated immediately following a prompt full diagnostic evaluation; delay between presentation and therapy increases the risk of a poor outcome (12) (evidence level 2b). There is no clear distinction in the clinical signs present when the infant has GBS sepsis compared with any other invasive organism.
When does invasive GBS occur?
Although invasive GBS disease does occur in infants whose mothers have negative screening cultures at 35 to 37 weeks, the risk is very low even in those with prolonged rupture of membranes or intrapartum pyrexia (28) (evidence level 2b). It is suggested that a limited diagnostic evaluation be performed in this newborn population (recommendation grade B).
Why is a WBC count not required for sepsis?
An infant with signs of sepsis does not require confirmatory tests other than obtaining cultures before commencing therapy, because no other tests have an adequately high negative predictive value to avoid therapy (evidence level 2a). In particular, a normal WBC count or differential should not prevent treatment in such an infant because the negative likelihood ratio of a normal CBC is approximately 0.7 (recommendation grade B) (16).
When should a GBS positive woman be discharged?
If a GBS-positive woman receives IAP less than 4 h before delivery (or receives no antibiotics or a nonpenicillin regimen), then a limited diagnostic evaluation is required, and the infant should not be discharged before 24 h of age. At the time of discharge, the infant should be evaluated and the parents should be educated regarding signs of sepsis in the newborn. Discharge at 24 h to 48 h is conditional on the parents’ ability to immediately transport the baby to a health care facility if clinical signs of sepsis develop (recommendation grade B).
