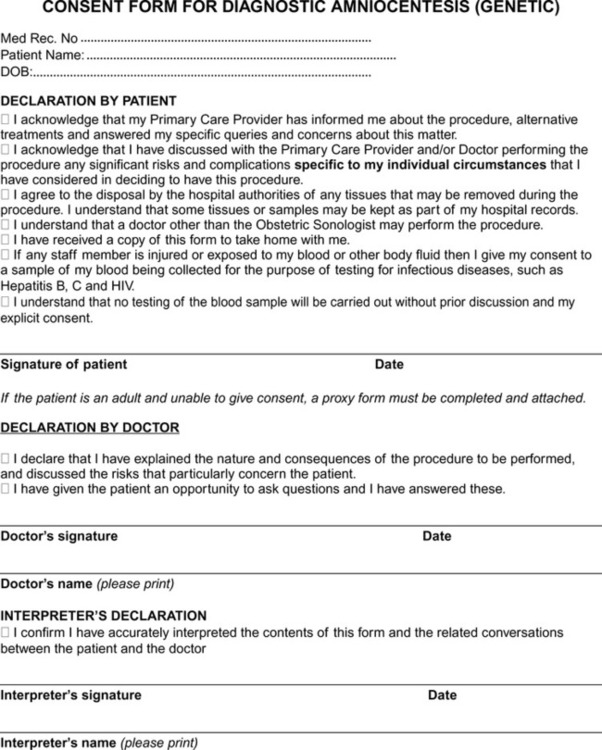
Formal permission granted by an individual to his/her carers to provide him/her care. Patients have the right to refuse any treatment they do not wish and the right to receive full information about a treatment, its purpose and possible side effects. Under normal circumstances if consent is not obtained, the treatment cannot be given.
What is patient consent to treatment?
Patient Consent to Treatment. Doctors give information about a particular treatment or test in order that a patient can decide whether or not to undergo such treatment or test. This process of understanding the risks and benefits of treatment is known as informed consent. It is based on the moral and legal premise of patient autonomy. Usually,...
What is informed consent in healthcare?
Informed consent is when a healthcare provider — like a doctor, nurse, or other healthcare professional — explains a medical treatment to a patient before the patient agrees to it. This type of communication lets the patient ask questions and accept or deny treatment.
What are the essentials of a valid medical consent?
Medical ethics and international human rights law necessitate consent as a prerequisite for initiating medical treatment. The essentials of a valid consent are: The patient must be informed of all the information regarding the treatment before the application; and The patient must be capable of giving consent.
What are the requirements for medical treatment without consent?
The patient must be informed of all the information regarding the treatment before the application; and The patient must be capable of giving consent. However, in certain situations medical treatment can be initiated without consent.

What is statutory informed consent?
Informed consent occurs when there is agreement to an interaction or action rendered with knowledge of relevant facts, such as the risks involved or any available alternatives. Informed consent often comes up in the contexts of legal ethics, medical treatment, and waiver of constitutional rights.
What are the 4 types of medical consent?
There are 4 components of informed consent including decision capacity, documentation of consent, disclosure, and competency. Doctors will give you information about a particular treatment or test in order for you to decide whether or not you wish to undergo a treatment or test.
What are the two types of consent for medical treatment?
There are two types of consent that a patient may give to their medical provider: express consent and implied consent. Express consent is typically done in writing, while implied consent is typically conveyed through a patient's actions or conduct.
What are the three types of patient consent?
There are three common ways to collect informed consent from your patient before a medical procedure. You can request written consent, use an online form or ask for oral consent.
What are the 5 types of consent?
What are the Different Types of Consent?Informed consent.Implied consent.Explicit consent.Active consent.Passive consent.Opt-Out consent.Key takeaway.
What are different types of consent?
Consent can be given: verbally – for example, a person saying they're happy to have an X-ray. in writing – for example, signing a consent form for surgery....How consent is givennurse arranging a blood test.GP prescribing new medication.surgeon planning an operation.
What is the difference between consent and implied consent?
What's the Main Difference? The main difference between express and implied consent is the succinctness with which consent is expressed. It can be much more difficult to prove implied consent than express consent. But even written agreements can sometimes lack true consent.
What's the difference between consent and informed consent?
There is a difference between general consent and informed consent. General consent is required before the patient can be examined or treated or before minor testing (such as lab work or routine imaging studies) can be done. No explanation of the contact is necessary, but consent to touch the patient is required.
What is the difference between implied consent and informed consent?
There is no formal agreement. For example, a patient who calls to make an appointment is giving implied consent to treatment. While implied consent is informal, informed consent is a legal term that requires seven elements to be valid: The individual is competent and can understand what they're consenting to.
What are the 4 types of consent NHS?
Consent FormsConsent Form 1 – Patient agreement to investigation, treatment or procedure.Consent Form 2 – Parental agreement to investigation, treatment or procedure for a child or young person.Consent Form 3 – Patient Parental agreement to investigation, treatment or procedure where consciousness not impaired.More items...•
What are consent requirements?
Valid informed consent for research must include three major elements: (1) disclosure of information, (2) competency of the patient (or surrogate) to make a decision, and (3) voluntary nature of the decision.
What are the 5 elements of informed consent?
B. Basic Elements of Informed ConsentDescription of Clinical Investigation. ... Risks and Discomforts. ... Benefits. ... Alternative Procedures or Treatments. ... Confidentiality. ... Compensation and Medical Treatment in Event of Injury. ... Contacts. ... Voluntary Participation.
What is consent to treatment?
Patient Consent to Treatment. Every human being of adult years and sound mind has a right to determine what shall be done with his/her own body [i]. All types of medical treatment require a patient’s consent. Consent is the permission necessary to start treatment.
What is consent in medical terms?
Consent is the permission necessary to start treatment. Medical ethics and international human rights law necessitate consent as a prerequisite for initiating medical treatment. The essentials of a valid consent are: Consent must be voluntarily made; The patient must be informed of all the information regarding the treatment before the application;
What is consent given without knowing its dangers?
A consent given without knowing its dangers and the degree of danger, is a consent that does not represent a choice and is inadequate [vi]. Only the physician giving treatment or performing an operation has a duty to inform the patient of the risks involved.
What is express consent?
Express consent is given to carry out a specific action. Implied consent can be inferred from their actions, the facts and circumstances of a particular situation. Implied consent can be obtained from a patient’s silence. There is no legal requirement to obtain written consent from a patient for medical treatment.
Why do doctors give information about a particular treatment?
Doctors give information about a particular treatment or test in order that a patient can decide whether or not to undergo such treatment or test. This process of understanding the risks and benefits of treatment is known as informed consent. It is based on the moral and legal premise of patient autonomy.
Which state requires a physician to obtain the signature of the patient to a statement containing an explanation of the procedure
For example the state of Nevada requires a physician to obtain the signature of the patient to a statement containing an explanation of the procedure, alternative methods of treatment, and risks involved [iv]. The principles governing consent for medical treatment are: consent must be valid;
Is consent necessary in an emergency?
Moreover, in case of an emergency, consent is not necessary [ii]. In case of an emergency, a surgeon can operate on a child without waiting for authority from the parents where it appears impracticable to secure consent [iii]. Consent can be either explicit or implied.
What is consent form?
This form is a legal document that shows your participation in the decision and your agreement to have the procedure done.
What does informed consent mean?
If you decide to move forward, you’ll need to give informed consent first. Informed consent means that you made a voluntary and educated decision. It also means that your healthcare provider has fully explained the medical procedure, including its risks and benefits.
Why is informed consent required in research?
It informs the participants about the trial and lets them make educated decisions about taking part in the study. The process is similar to informed consent in healthcare. In a research setting, it involves discussing the following:
What does it mean when you sign a medical form?
When you sign the form, it means: You received all the relevant information about your procedure from your healthcare provider. You understand this information. You used this information to determine whether or not you want the procedure. You agree, or consent, to get some or all of the treatment options.
What is the role of medical information in decision making?
your understanding of the medical information. your voluntary decision to get treatment. These components are essential elements of the shared decision-making process between you and your healthcare provider. Most importantly, it empowers you to make educated and informed decisions about your health and medical care.
Is implied consent written down?
It isn’t explicitly stated or written down. For example, if you have a fever and see a healthcare provider, your visit implies that you want treatment. Another example is if you break an ankle and visit a healthcare provider for crutches. Compared to informed consent, implied consent is less formal.
Can you give consent to someone else?
This allows someone else to give consent on your behalf if you’re unable to. You can’t give consent. Another person can make your medical decisions if you can’t provide consent. This may happen if you’re in a coma, or have a condition like advanced Alzheimer’s disease.
When should informed consent form be included in medical records?
Document the informed consent conversation and the patient’s (or surrogate’s) decision in the medical record in some manner. When the patient/surrogate has provided specific written consent, the consent form should be included in the record. In emergencies, when a decision must be made urgently, the patient is not able to participate in decision ...
What is the process of informed consent?
The process of informed consent occurs when communication between a patient and physician results in the patient’s authorization or agreement to undergo a specific medical intervention.
Why is informed consent important?
Informed consent to medical treatment is fundamental in both ethics and law. Patients have the right to receive information and ask questions about recommended treatments so that they can make well-considered decisions about care. Successful communication in the patient-physician relationship fosters trust and supports shared decision making.
What information should a physician include in a medical record?
The physician should include information about: The burdens, risks, and expected benefits of all options, including forgoing treatment. Document the informed consent conversation and the patient’s (or surrogate’s) decision in the medical record in some manner.
Can a surrogate be used as a physician without consent?
In emergencies, when a decision must be made urgently, the patient is not able to participate in decision making, and the patient’s surrogate is not available, physicians may initiate treatment without prior informed consent.
What is consent form?
General consent form information. Before a planned surgical procedure, the surgeon will ask you (or your legal guardian) to sign a consent form. The doctor, not the nurse, must obtain the patient’s consent. The form will have information specifically about the procedure.
What information should be given to a patient before making a decision about medical treatment?
The information that must be given to you as a patient includes: The diagnosis and likely outcome (prognosis) of your condition.
What is the right to be told about your medical condition?
You have a legal right to be told any information that relates to your medical condition and treatment. Without this information, you are not able to make a fully informed choice and give valid consent for treatment. Your doctor has a duty to explain your medical condition, the recommended treatment (including the other treatment options available) ...
How to take an active role in your own treatment?
Some ways to take an active role in your own treatment include: Find out as much as you can about the procedure. The best way to be actively involved in your care is to learn about the procedure, as well as about its risks and possible complications. Find out beforehand if something could go wrong.
What should a doctor talk about before treatment?
The doctor should talk to you about any special things you need to do before treatment and during recovery time. The success of your treatment may depend on following these instructions. Make sure that you understand the advice and are prepared and able to follow it.
Abstract
When consent to medical treatment is described as ‘valid’, it might simply mean that it has a sound basis, or it could mean that it is legally valid. Where the two meanings are regularly interchanged, however, it can lead to aspects of the sound basis or the legal requirements being neglected.
Introduction
At common law, the informational component of consent to treatment is different in relation to ‘valid consent’ (or ‘real consent’) and ‘informed consent’. 1 A failure to obtain valid consent can result in criminal assault and a civil claim of trespass to the person (battery) whereas a failure to obtain informed consent can constitute negligence.
Valid consent
Consent is on a sound basis if it complies with ethical principles and with the law. Common law and ethics require that consent is voluntary, that it is made by a person with capacity and that it is adequately informed. Since Montgomery in 2015, the requirement of informed consent is ‘firmly part of English law’.
Guidelines
Setting aside these specialist situations governed by statute, there are numerous guidelines that set out the requirements of consent at common law.
Does the label matter?
If the law requires that consent is valid and also that it is informed, then professional guidance is right to require both of these elements. It has been pointed out that some do so under the term valid consent and that this is not technically legally accurate. However, the law does not have a monopoly on the use of the term ‘valid’.
Conclusion
Informed consent is not necessarily valid (if it is not voluntary or capacitous) and consent that is valid is not necessarily adequately informed. This flows from the different informational thresholds that apply in battery and negligence. This is not always made apparent in the plethora of guidance on consent.
Acknowledgments
Thanks to Shaun Pattinson for comments on a previous draft and to the anonymous reviewers.
