Biological Nitrification Process in Waste Water Treatment System
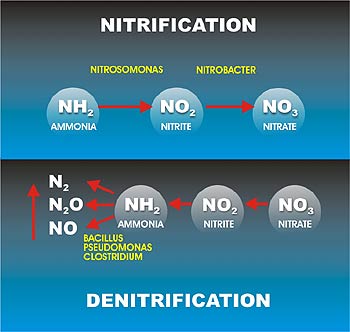
- Definition. The removal of nitrogen by biological nitrification and denitrification is a two-step process. ...
- Purpose of Nitrification. Total concentration of organic and ammonia nitrogen in municipal wastewater in the range 25‐ 45 mg/L as nitrogen based on flowrate of 450 L/capita.d (120 gal/capita.d)
- Nitrification Process. ...
What are the steps in the process of nitrification?
Biological nitrification is the microbe-mediated process of oxidizing ammonia to remove nitrogenous compounds from wastewaters. Domestic sewage typically contains 20 to 40 mg/L (ppm) of ammonia nitrogen (NH 4-N). Organic matter containing nitrogen, e.g., protein and nucleic acid, also biodegrades to release ammonia.
What is filtration process in water treatment?
nitrification. Nitrification is defined as the biological or biochemical removal (oxidation) of ammonia (NH4 +) by certain bacteria in the presence of oxygen. This special group of bacteria are called “nitrifiers,” “nitrifying bacteria,” or “nitrification bacteria.” Wastewater generated by the
What is biotower in waste water treatment?
Nitrification is the process of oxidizing ammonia to remove nitrogenous compounds from wastewater. Domestic sewage typically contains 20 to 40 mg/L (parts per million) of ammonia nitrogen, and it takes 4.5 mg of oxygen to fully oxidize 1.0 mg of ammonia nitrogen.
What is the process of nitrification?
Nitrification is a microbial process by which reduced nitrogen compounds (primarily ammonia) are sequentially oxidized to nitrite and nitrate. Ammonia is present in drinking water through
What is nitrification simple terms?
Definition of nitrification : the oxidation (as by bacteria) of ammonium salts to nitrites and the further oxidation of nitrites to nitrates.
What happens in nitrification?
Nitrification is a microbial process by which reduced nitrogen compounds (primarily ammonia) are sequentially oxidized to nitrite and nitrate. Ammonia is present in drinking water through either naturally-occurring processes or through ammonia addition during secondary disinfection to form chloramines.
What is nitrification and why is it important?
Nitrification is an aerobic microbial process by which specialized bacteria oxidize ammonium to nitrite and then to nitrate. Nitrification is a very important part of the nitrogen cycle , because for most plants nitrate is the preferred chemical form of nitrogen uptake from soil or water .
What affects nitrification and wastewater?
Main factors affecting nitrification are temperature, pH, alkalinity (carbonate and bicarbonate concentration), dissolved oxygen (DO) and ammonia nitrogen (NH4-N) concentration, organic matter / ammonia nitrogen ratio, and the presence of inhibiting substances (free ammonia (FA), free nitrous acid, free hydroxylamine ...
What is nitrification describe its mechanism?
Nitrification is a microbial process by which reduced nitrogen compounds (primarily ammonia) are sequentially oxidized to nitrite and nitrate. Ammonia is present in drinking water through either naturally-occurring processes or through ammonia addition during secondary disinfection to form chloramines.
Which bacteria is used for nitrification?
The nitrification process requires the mediation of two distinct groups: bacteria that convert ammonia to nitrites (Nitrosomonas, Nitrosospira, Nitrosococcus, and Nitrosolobus) and bacteria that convert nitrites (toxic to plants) to nitrates (Nitrobacter, Nitrospina, and Nitrococcus).Feb 21, 2022
What is the advantage of nitrification?
The benefits include protecting the quality of the receiving water, permit compliance, strengthening of the floc particles, control of undesired filamentous growth, return of alkalinity to the treatment process, and cost-savings for the treatment or degradation of cBOD.
What is needed for nitrification?
Typically, the amount of residual alkalinity required to maintain pH near neutral is between 70 and 80 mg/L as CaCO3. Alkalinity is a major chemical requirement for nitrification and can be a useful and beneficial tool for use in process control.
What conditions are needed for nitrification?
The major factors are soil temperature, pH, water content, and the presence of oxygen. Other factors that can influence the process include soil salinity, texture, and the N source. Soil nitrifying bacteria are generally more sensitive to envi- ronmental stresses than many other soil bacteria.
Why was there denitrification before nitrification?
Nitrification is an essential process as it helps to provide nitrates to plants which act as a source of nitrogen. Denitrification is an important process that ensures the cyclic movement of nitrogen from the atmosphere to the soil, plants, and back to the atmosphere.Jan 2, 2022
What is meant by nitrification and denitrification?
In Nitrification, nitrifying bacteria oxidise ammonia to nitrite and then it is further oxidised to nitrate. Nitrate is thus made available for plants to absorb. Denitrification is the opposite of nitrification. In denitrification, microorganisms reduce nitrate back to nitrogen.
What is denitrification used for?
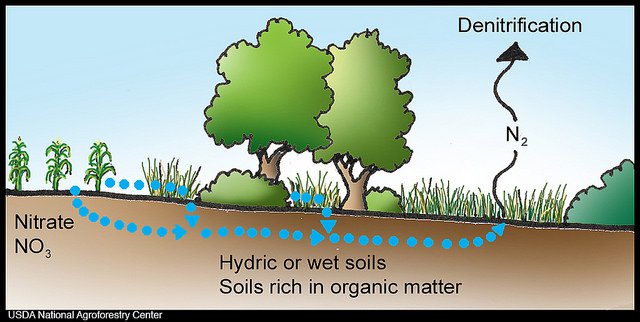
Denitrification is commonly used to remove nitrogen from sewage and municipal wastewater. It is also an instrumental process in constructed wetlands and riparian zones for the prevention of groundwater pollution with nitrate resulting from excessive agricultural or residential fertilizer usage.
Which bacteria are responsible for nitrification?
Aerobic autotrophic bacteria are responsible for nitrification in activated sludge and biofilm processes; Two‐step process in nitrication involve two groups of bacteria; First stage, ammonia is oxidized to nitrite by one group (Nitrosomonas) and second stage, nitrite is oxidized to nitrate by another group of autotrophic bacteria (Nitrobacter) ...
What is the process of removing nitrogen?
Definition. The removal of nitrogen by biological nitrification and denitrification is a two-step process. In the first step (nitrification), ammonia is converted aerobically to nitrate (NO 3− ). In the second step (denitrification), nitrates are converted to N 2 O or nitrogen gas (N 2) under anoxic conditions.
What is nitrification in wastewater?
Nitrification is one of the two primary mechanisms for ammonia removal in aerobic wastewater systems. Nitrification is a two-step process performed by two categories of bacteria, ammonia oxidizers and nitrite oxidizers. Ammonia oxidizing bacteria (AOB) convert ammonia to nitrite through the ammonia oxidation pathway.
Why is alkalinity important for nitrification?
The first is nitrifiers use dissolved carbon dioxide, carbonate, and bicarbonate as their carbon source for autotrophic production of glucose, and (CO2, HCO3-, CO3-2) are major contribu tors to a system’s alkalinity. The second reason is nitrifiers produce nitric acid during ammonia oxidation and if alkalinity is low, pH fluctuations due to acid production can lead to poor nitrifier growth. This means in cases of low alkalinity, it is much more beneficial to supplement alkalinity with carbonate or bicarbonate, rather than other commonly added basic compounds such as sodium hydroxide or magnesium hydroxide. Typically, an alkalinity around 100 ppm is optimal for nitrifier function but there is no harm in being lower or higher assuming the system is maintaining a stable pH and nitrification is functioning well enough to handle the rate of ammonia loading in a system. 8.64 mg/L bicarbonate (HCO3) is considered adequate to remove 1 ppm of ammonia in wastewater systems based on a model of nitrification from 1976 (USEPA, 2002).
What is the primary form of ammonia removal?
Nitrification becomes the primary form of ammonia removal after levels of BOD have been exhausted. Ammonia is often released in the decomposition of urea but will also appear in lower quantities due to the degradation of proteins and other nitrogen-containing molecules. Organic forms of nitrogen, such as amino acids and proteins, tend to be favored forms of nitrogen for heterotrophic bacteria. This means in wastewater treatment systems some excess ammonia is often present that must be oxidized by nitrifiers to achieve effluent limits for ammonia. In cases of lost nitrification , this extra ammonia is what is observed in waste discharge.
Does temperature affect nitrification?
Rapid temperature changes also have significant adverse effects on nitrification since nitrifiers are unable to adjust quickly due to their slow growth rates. Nitrifiers have been observed functioning over 35°C (as high as 52°C) in a recent study (Knight, 2019), which had been thought to be near impossible. 3.
What temperature do nitrifiers need to grow?
Nitrifiers prefer a temperature range between 15–30°C. Nitrifiers have trouble growing fast enough to maintain a population below 15°C, and sometimes have problems with low dissolved oxygen levels above 30°C as oxygen solubility decreases in higher temperatures. Nitrifiers have been recorded functioning effectively outside this temperature range as the bacterial population is often able to acclimate to varied temperatures. Rapid temperature changes also have significant adverse effects on nitrification since nitrifiers are unable to adjust quickly due to their slow growth rates. Nitrifiers have been observed functioning over 35°C (as high as 52°C) in a recent study (Knight, 2019), which had been thought to be near impossible.
What percentage of bacteria are nitrifying?
Nitrifying bacteria are typically thought to make up 0.39–9% of bacterial populations in activated sludge (Yao & Peng, 2017). Most nitrifying activated sludge systems have 4–6% of the bacterial population made up by ...
How much of the population of nitrifying bacteria is activated sludge?
Nitrifying bacteria make up 4-6% of the population in an activated sludge process and have limited diversity compared to heterotrophic bacteria in wastewater. This makes them more susceptible to toxicity.