
Is there a new treatment for multiple sclerosis?
More research is needed to study its potential benefits and risks for treating MS. Hematopoietic stem cell transplantation (HSCT) therapy is a promising new treatment for MS that’s currently being studied. It’s not currently approved, but interest is growing in the field, and it’s being evaluated in clinical trials.
What is the focus of new research on multiple sclerosis?
The focus of research has shifted in recent years. T cells, a type of cell in your body’s immune system, were long thought to be responsible for MS. But recent studies show that another type of immune cell, called B cells, also play a role in the attack on your myelin. New treatments target these cells.
What are my options for treating MS?
Just over 20 years ago there were no drug treatment options for MS at all, but today there is a wide variety of FDA-approved medications. Disease modifying agents, such as beta interferons and newer oral drugs alter the immune system to slow disease progression and reduce attacks.
Which interferons are approved for relapsing multiple sclerosis (MS)?
The beta interferons that are approved for the relapsing forms of MS include: 1 Avonex - interferon beta-1a 2 Rebif - interferon beta-1a 3 Betaseron - interferon beta-1b 4 Extavia - interferon beta-1b
How close are we to a cure for multiple sclerosis?
Although there is no cure for MS, we can see a future where people can live free from its effects and not worry about their MS getting worse. There are now a number of health conditions - like rheumatoid arthritis or Type 1 diabetes – where there are no cures.
What is the most effective multiple sclerosis treatment?
Interferon Beta (Avonex, Betaseron, Extavia, Plegridy, Rebif) How it works: These are lab-made versions of your body's infection-fighting protein. They've been around the longest and are the most widely prescribed drugs for MS.
What are some current experimental treatments for MS?
Experimental Treatments for Progressive MS Masitinib, simvastatin, ibudilast, and lipoic acid are currently being tested in clinical trials as potential therapies for progressive forms of MS.
Is Ocrevus better than Tysabri?
Results showed that annual relapse rates were lower for Tysabri than Ocrevus, and patients on Tysabri were significantly less likely to have had any relapse after 12 or 24 months of treatment. Further analyses indicated that patients on Tysabri were at an approximately 30% lower risk of any relapse.
Which is better Ocrevus vs Kesimpta?
The safety and efficacy data of Ocrevus is, overall, comparable with that of Kesimpta. The main differentiation between the two drugs is the delivery system and the annual cost of therapy, which is $65,000 for Ocrevus compared to $83,000 for Kesimpta in the US.
Which is better Ocrevus or Gilenya?
Ocrevus (ocrelizumab) may be more effective than Gilenya (fingolimod) at preventing relapse in relapsing-remitting multiple sclerosis (RRMS) patients who recently transitioned from Tysabri (natalizumab), according to a new study.
What is the latest research on MS?
Researchers Develop New Antibody Test to Diagnose Multiple Sclerosis. Mar. 24, 2022 — Researchers have validated a new antibody test to diagnose multiple sclerosis (MS), a potentially disabling disease of the brain and spinal ...
Why is Benadryl great for multiple sclerosis?
In light of previous laboratory studies of the antihistamine compound at UCSF, the researchers said, the drug most likely exerted its effect by repairing damage MS had inflicted on myelin, an insulating membrane that speeds transmission of electrical signals in the nervous system.
Can stem cells cure multiple sclerosis?
While there is no cure for MS, stem cell therapy can help improve a person's symptoms and slow down the progression of the disease. Stem cell therapy is an experimental treatment that people can access through clinical trials. MS causes the body to direct an immune response to its own central nervous system.
How many years can you stay on Ocrevus?
This study is recruiting 600 people with either secondary or primary progressive MS. All participants will take Ocrevus every 24 weeks for four years. Progression of disability will be assessed using a combination of measures.
Is Mavenclad better than Ocrevus?
Ocrevus is also used to treat primary progressive forms of multiple sclerosis (MS). Because of its safety profile, use of Mavenclad is generally recommended for patients who have had an inadequate response to, or are unable to tolerate, an alternate drug indicated for the treatment of MS.
Will Ocrevus cure MS?
It targets a type of immune cell called a CD20-positive B cell that plays a key role in the disease. OCREVUS is approved by the FDA to treat relapsing or primary progressive forms of multiple sclerosis (MS).
What is the FDA approved DMT?
To date, the Food and Drug Administration (FDA) has approved more than a dozen DMTs for different types of MS. Most recently, the FDA has approved: Ocrelizumab (Ocrevus). It treats relapsing forms of MS and primary progressive MS (PPMS). It’s the first DMT. Fingolimod (Gilenya).
When was DMT approved?
It’s the newest DMT to be added to the market and was FDA approved in March 2020. Ponesimod (Ponvory). The FDA approved this drug in March 2021. Ponvory has been shown to reduce annual relapses for relapsing types of MS by 30.5 percent when compared with teriflunomide (Aubagio).
Does ibudilast help with MS?
The results of a phase 2 clinical trial suggest that ibudilast might help reduce the progression of disability in people with MS. To learn more about this medication, the manufacturer plans to conduct a phase 3 clinical trial.
Is HSCT available over the counter?
This oral antihistamine is currently available over the counter, but not in the dose used in the clinical trial. More research is needed to study its potential benefits and risks for treating MS. Hematopoietic stem cell transplantation (HSCT) therapy is a promising new treatment for MS that’s currently being studied.
Is there a cure for MS?
There’s currently no cure for multiple sclerosis (MS), but treatment can help manage it. In recent years, new medications have become available to help slow the progression of the disease and relieve symptoms. Researchers continue to develop new treatments and learn more about the causes and risk factors of this disease.
Does clemastine fumarate help with MS?
The findings of a small 2017 study suggest that clemastine fumarate might help restore the protective coating around nerves in people with relapsing forms of MS.
What is the best treatment for myelin?
Treatments under the microscope include: An antihistamine (a type of drug often used for allergies) called clemastine fumarate ( Tavist ). It was found to improve nerve signals in people with damage to their optic nerves. This suggests that some myelin was rebuilt. Metformin, a common diabetes drug.
What is a new blood test for MS?
For example, a simple new blood test that measures small amounts of neuron-derived proteins (neurofilaments) may be used to predict how serious your MS will be and how well your treatment will protect brain tissue. Scientists continue to study known biomarkers for more information and to look for new ones.
How often do you get ocrelizumab?
The National Multiple Sclerosis Society calls ocrelizumab a "game changer.". You get it in an IV every 6 months. Another drug that targets B cells has been approved by the FDA for relapsing MS, the most common type.
What are the genes that cause MS?
There are genes known as "susceptibility genes” that seem to raise your risk for MS. Scientists are studying how these genes work in the nervous system in order to learn more about how they could lead to MS. This includes studies about the way genes that underlie gender affect MS.
What type of immune system is responsible for MS?
The focus of research has shifted in recent years. T cells, a type of cell in your body’s immune system, were long thought to be responsible for MS. But recent studies show that another type of immune cell, called B cells, also play a role in the attack on your myelin. New treatments target these cells.
What is a DMT for MS?
Disease-modifying therapies (DMTs) are drugs used to prevent MS from getting worse. The American Academy of Neurology recommends that people start using a DMT soon after diagnosis to protect their myelin. This can: Reduce the number of new MS attacks, called relapses.
Does ofatumumab reduce MS?
It also cut the risk of disability getting worse by about 30%. And it reduced the number of new MS lesions (scars). Other recent treatment highlights include:
What is the best treatment for multiple sclerosis?
Official Answer. The newest drugs for the treatment of multiple sclerosis include Ponvory, Kesimpta, Bafiertam, Zeposia, Vumer ity, Mavenclad, Mayzent, Ocrevus, and Lemtrada. Multiple sclerosis (MS) is a neurological disease that affects that affects the brain and spinal cord. In MS, the body's immune system attacks the protective myelin sheaths ...
Is MS a lifelong illness?
MS is a lifelong illness. It can follow one of several different patterns: Relapsing-Remitting MS (RRMS). The most common form of multiple sclerosis characterized by temporary periods of relapses and remissions. Secondary-Progressive MS (SPMS).
What is the FDA approved medication for MS?
Cladribine (Mavenclad) is another oral tablet approved by the FDA in 2019 to treat relapsing-remitting and secondary-progressive forms of MS. In clinical trials, cladribine reduced the progression of disability and significantly reduced relapse rates.
How many people have progressive MS?
About 10% of people with multiple sclerosis are diagnosed with a progressive form (primary-progressive MS) at the onset of the disease.
Why is cladribine used for MS?
Ocrelizumab (Ocrevus) was approved by the FDA in 2017. This drug reduces relapse rate and risk of disability progression in relapsing-remitting MS.
Can you transition to secondary progressive MS?
Some people with relapsing-remitting MS can transition to seconda ry-progressive MS after several years. Currently available DMTs have little impact on this phase of MS, so it's best to develop a treatment regimen during the earlier relapsing-remitting phase.
Is there a cure for MS?
There is no cure for multiple sclerosis (MS), but there has been much progress in developing new drugs to treat it. Research is ongoing to develop new and better disease-modifying therapies (DMTs) for this disease of the central nervous system.
What is the treatment for multiple sclerosis?
skin infections. Kesimpta, (ofatumumab), a CD20-directed cytolytic antibody was also approved in August 2020. It is used for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
What is MS treatment?
What is Multiple Sclerosis (MS)? There's good news if you're living with multiple sclerosis (MS): new and easier treatments for MS are being approved at record speeds. In fact, since 2019, six new options have cleared the FDA. Multiple sclerosis (MS) is a disease that affects the brain, spinal cord, and optic ...
What is the best oral medicine for MS?
New Oral MS Medications. Beta interferon preparations or glatiramer (Copaxone) may be the initial multiple sclerosis (MS) therapy chosen by many doctors. The "ABC" drugs (Avonex , Betaseron , and Copaxone ) are often the three first-line agents used for long-term treatment of multiple sclerosis (MS). However, side effects and the inconvenience ...
What is the treatment for MS attacks?
Attacks themselves often require different treatments. For example, corticosteroids like oral prednisone or IV methylprednisolone (Solu-Medrol) may be used to reduce inflammation in MS. Plasma exchange ( plasmapheresis) has been used to treat severe symptoms in patients who do not respond to corticosteroids.
What is the diagnosis of MS?
Diagnosis of MS involves a clinical exam by the physician (neurologist). Diagnostic tests such as a magnetic resonance imaging (MRI) of the brain and spinal cord will be performed. An evaluation of the cerebrospinal fluid (CSF) and certain blood tests may also take place.
What is the name of the disease that affects the brain, spinal cord, and optic nerve?
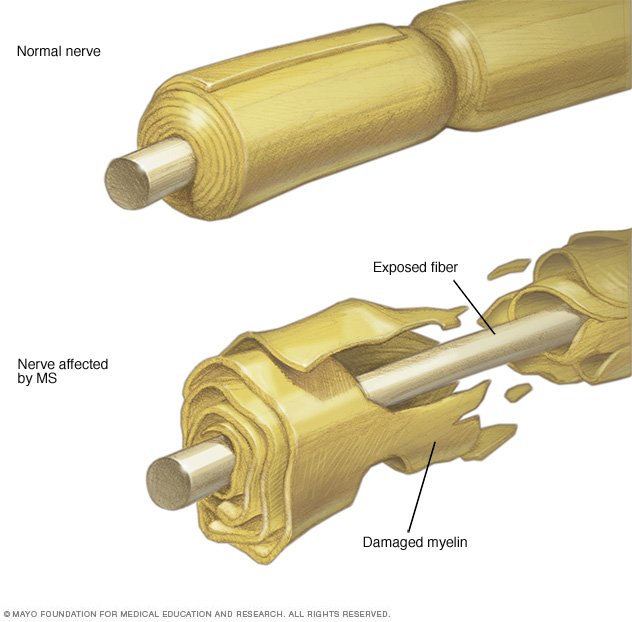
Multiple sclerosis (MS) is a disease that affects the brain, spinal cord, and optic (eye) nerve -- all parts of the central nervous system (CNS). MS has features of a disease in which the body's immune system attacks the myelin sheaths, which are the protective covering of the nerves. When the myelin is damaged and forms scar tissue -- also called ...
When was Betaseron approved?
Betaseron was approved by the FDA for relapsing‐remitting MS in 1993 , becoming the first available drug that affected the underlying disease. Disease modifying agents, such as beta interferons and newer oral drugs alter the immune system to slow disease progression and reduce attacks.
