Which monoclonal antibody is best?
Bebtelovimab is a monoclonal antibody therapy with EUA for the treatment of mild to moderate COVID-19 in individuals with positive results of direct SARS-CoV-2 viral testing who are at high risk for progression to severe COVID-19, including hospitalization or death and for whom alternative COVID-19 treatment options approved or authorized by ...
What are the dangers of monoclonal antibodies?
Bebtelovimab is Florida’s only available monoclonal antibody treatment. On January 24, 2022, the U.S. Food and Drug Administration (FDA) revised the Emergency Use Authorizations (EUA) for Eli Lilly’s bamlanivimab-etesevimab and Regeneron’s REGEN-COV monoclonal antibody treatments , discontinuing their use by any U.S. state or jurisdiction.
How effective is the monoclonal treatment?
Tocilizumab (EUA issued June, 24 2021) Bebtelovimab (EUA issued February 11, 2022) The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply: The patient has a …
Are there side effects of monoclonal antibody treatment?
Jan 22, 2021 · Locations across the U.S. where monoclonal antibody therapy is offered can be found using the online locator at the HHS website, where users will see a map of locations where the treatment is...

What are monoclonal antibodies used for during the COVID-19 pandemic?
Monoclonal antibodies are laboratory-made proteins that mimic the immune system's ability to fight off harmful pathogens such as viruses, like SARS-CoV-2. And like other infectious organisms, SARS-CoV-2 can mutate over time, resulting in certain treatments not working against certain variants such as omicron.Jan 24, 2022
Is there a monoclonal antibody therapy for post COVID-19 exposure?
FDA authorizes bamlanivimab and etesevimab monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19 | FDA.Sep 16, 2021
What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
Do you need a prescription for Paxlovid?
Can anyone get a Paxlovid prescription? The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. But in order to qualify for a prescription, you must also have had a positive COVID-19 test result and be at high risk for developing severe COVID-19.Apr 12, 2022
Can I get the COVID-19 vaccine if I was treated with monoclonal antibodies or convalescent plasma?
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
Can I get COVID-19 again after having the vaccine?
Getting COVID-19 after you've been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you.Nov 9, 2021
Who should not take the Pfizer-BioNTech COVID-19 vaccine?
If you have had a severe allergic reaction to any ingredient in the Pfizer-BioNTech COVID-19 vaccine (such as polyethylene glycol), you should not get this vaccine. If you had a severe allergic reaction after getting a dose of the Pfizer-BioNTech COVID-19 vaccine, you should not get another dose of an mRNA vaccine.
How can I get Paxlovid?
5. Can anyone get a Paxlovid prescription? The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. But in order to qualify for a prescription, you must also have had a positive COVID-19 test result and be at high risk for developing severe COVID-19.Apr 12, 2022
Is the antiviral medication Paxlovid authorized for COVID-19?
On Dec 22, 2021, the US Food and Drug Administration (FDA) issued an emergency use authorisation for Pfizer's COVID-19 antiviral, Paxlovid.Jan 13, 2022
Is there an emergency use authorization of Paxlovid for COVID-19 in the US?
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the emergency use of the unapproved product PAXLOVID for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kg)Apr 14, 2022
Monoclonal Antibody Therapy
Glaxo Smith Kline’s monoclonal antibody Sotrovimab is authorized for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in:
Preventative Monoclonal Antibody Therapy: EvuSheld
Preventative monoclonal antibody therapy locations are marked with a blue pin on the locator map.
COVID-19 VEKLURYTM (remdesivir)
Following the recent statement from the National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel about therapies for the COVID-19 Omicron variant, CMS created HCPCS code J0248 for VEKLURY™ (remdesivir) antiviral medication when administered in an outpatient setting.
COVID-19 Monoclonal Antibody Products
The FDA authorized the following investigational monoclonal antibody product under EUA for pre-exposure prophylaxis of COVID-19:
Important Update about Viral Variants
On April 16, 2021, the FDA revoked the EUA for bamlanivimab, when administered alone , due to a sustained increase in COVID-19 viral variants in the U.S. that are resistant to the solo product.
Medicare Coverage for COVID-19 Monoclonal Antibody Products
During the COVID-19 public health emergency (PHE), Medicare will cover and pay for these infusions (when furnished consistent with their respective EUAs) the same way it covers and pays for COVID-19 vaccines.
Coding for the Administration of COVID-19 Monoclonal Antibody Products
CMS identified specific code (s) for each COVID-19 monoclonal antibody product and specific administration code (s) for Medicare payment:
Medicare Payment for Administering COVID-19 Monoclonal Antibody Products
To ensure immediate access during the COVID-19 PHE, Medicare covers and pays for these infusions and injections in accordance with Section 3713 of the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) .
Billing for Administering COVID-19 Monoclonal Antibody Products
Health care providers can bill on a single claim for administering COVID-19 monoclonal antibody products, or submit claims on a roster bill.
What is monoclonal antibody therapy?
Monoclonal antibodies are laboratory-produced molecules that may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. These antibodies could help the immune system respond more effectively to the virus.
How old do you have to be to get a monoclonal antibody?
age 12 years and older. Patients who have a positive COVID-19 test should ask their healthcare provider if they are eligible for monoclonal antibody treatment and where they can go to receive the infusion. If you have questions, contact the Combat COVID Monoclonal Antibodies Call Center at 1-877-332-6585. YouTube.
Where to get monoclonal antibody therapy
Locations across the U.S. where monoclonal antibody therapy is offered can be found using the online locator at the HHS website, where users will see a map of locations where the treatment is available.
The wider picture
The novel coronavirus has infected more than 97.6 million people, including just over 24.6 million in the U.S., since it was first reported in Wuhan, China.
What antibody is used to block the virus?
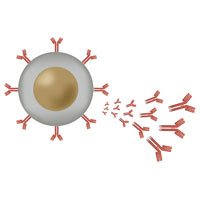
Monoclonal antibodies against COVID-19 attach to the virus to block it from entering human cells. The monoclonal antibody protein also “marks” the virus to be broken down by the immune system and cleared from the body.
What is the function of antibodies?
Antibodies are proteins that exist in our bodies as part of our immune system to recognize and defend against harmful viruses and bacteria. Monoclonal antibodies are made in a laboratory and designed to target a specific virus or bacteria.
Can monoclonal antibodies cause nausea?
Most people tolerate monoclonal antibody infusions very well. Some people may experience infusion-related side effects, such as nausea and dizziness, that are short-lived and go away on their own. As with any medication, there is the potential for mild or more severe allergic reactions, which are uncommon.
What are COVID-19 therapeutic treatments?
MONOCLONAL ANTIBODY TREATMENTS Your body naturally makes antibodies to fight infection. However, your body may not have sufficient antibodies to recognize a new virus like the one that causes COVID-19.
Who should get covid-19 treatments?
If you are age 65 or older or have a high-risk medical condition and have mild to moderate symptoms, reach out to your health care provider to ask about monoclonal antibody treatment — as soon you get your positive test result. The treatment works best in the first five days and can reduce the chance of being hospitalized by 70%.
How to get COVID-19 Therapeutics
There are different types of treatments available. Your health care provider will help determine which one is right for you.
How do monoclonal antibodies work?
Depending on the treatment your receive, the process takes about 2 to 3 hours.
PREVENTIVE MEDICATION
For people who are immunocompromised or who are unable to receive the vaccine, there is a long-acting monoclonal treatment available that has received Emergency Use Authorization called Evusheld. This drug is administered through an injection and has shown a 77% reduction in developing COVID-19 symptoms.
What is Monoclonal Antibody Therapy?
Monoclonal antibodies are designed to fight a specific disease or cell. In cancer treatment, for example, monoclonal antibodies can be used in conjunction with traditional cancer treatments like chemotherapy to directly target cancer cells and help manage symptoms of advanced stages of certain types of cancer.
Is it FDA Approved?
Sorta, in 2020 the Food and Drug Administration issued an emergency use authorization for monoclonal antibody treatment for COVID-19. This was issued as an emergency use authorization which means it is not fully FDA approved.
Are There Any Side Effects?
Monoclonal Antibody Therapy is used to neutralize Covid, therefore eliminating it from your body. Patients may experience mild headaches, low energy, and dizziness. However, they are not long-lasting and are easily treated with over-the-counter remedies such as pain killers and vitamin supplements.
Why Would I Need Monoclonal Antibody IV Therapy?
There is no known cure for Covid, but doctors may recommend certain treatments to improve health and relieve symptoms. Monoclonal Antibody IV Therapy is a promising treatment for Covid.
Where Can I Get Monoclonal Antibody Therapy In California?
You may think that you’ll need to go to a big city in California or a hospital or clinic for Monoclonal Antibody Therapy, but that’s not true. This treatment is available right where you are. Drip Hydration offers its services to residential and commercial properties so you can get IV therapy delivered wherever your heart desires.
How Does In-Home Monoclonal Antibody Treatment Work?
In-home IV therapy allows you to receive your treatment at home. This can be especially helpful for those who have jobs or other responsibilities and cannot leave their house for long periods of time. It is administered by a medical professional.
Get Monoclonal IV Therapy For Covid-19 At Home
Monoclonal IV therapy for Covid-19 is an effective FDA-approved treatment that reduces the severity of SARS-CoV-2 symptoms. If you or a loved one is at risk of developing severe illness post-infection, we recommend getting an IV infusion to boost the body’s ability to fight infection.
Monoclonal Antibody (mAb) Treatment
To see if an infusion is right for you, contact your healthcare provider.
What is a monoclonal antibody?
A monoclonal antibody is a laboratory-made protein that mimics your immune system’s ability to fight off harmful viruses that can cause disease. It is a treatment that may make your COVID-19 disease less severe and hasten your recovery. Monoclonal antibody treatment is not a substitute for a COVID-19 vaccine.
Who can receive a monoclonal antibody treatment?
If you test positive for COVID-19 and meet the following criteria, you may be eligible to receive a monoclonal antibody treatment:
Out-Of-Hospital Treatment Options For Covid-19
- Oral Antiviral Treatments
The FDA authorized two oral antivirals, Pfizer's Paxlovid and Merck's molnupiravir, for the treatment of COVID-19 in certain patients. - Monoclonal Antibody Treatments
COVID-19 monoclonal antibody therapeutics (mAb)are available for people ages 12 years or older who: 1. Have tested positive for COVID-19 and have had symptoms for 10 days or less 2. Are at high risk of becoming seriously ill, including those who have been recently exposed to someone …
Hospital Treatments For Covid-19
- There are treatments for hospitalized patients with severe cases of COVID-19 that have been approved or authorized for emergency use by the Food and Drug Administration (FDA). 1. Remdesiviris an antiviral drug approved by the FDA for the treatment of COVID-19 in hospitalized adults and hospitalized pediatric patients at least 12 years of age. It works by stopping SARS-Co…
Ensuring The Safety and Effectiveness of Treatments
- After a public health emergencywas declared for the COVID-19 pandemic, it was determined that the Food and Drug Administration (FDA) could authorize the emergency use of tests, treatments, and vaccines to reduce suffering, loss of life and restore the health and security of our country. 1. FDA has approved the use of one anitviral drug Veklury (remdesivir) to treat COVID-19. FDA has …