
Technically, the answer is obvious: a heat treatment at which the temperature does not change is isothermal, and vise versa. From the kinetic point of view, when the temperature changes throughout a treatment, heating/cooling rate may seriously affect thermodynamically dictated processes.
Full Answer
What is isothermal process?
Heat " In thermodynamics, an isothermal process is a type of thermodynamic process in which the temperature of the system remains constant: Δ T = 0.
What is an isotherm in thermodynamics?
Each curve is called an isotherm. Such graphs are termed indicator diagrams and were first used by James Watt and others to monitor the efficiency of engines. The temperature corresponding to each curve in the figure increases from the lower left to the ht.
What is the meaning of heat treatment?
Process of heating something to alter it. Heat treating furnace at 1,800 °F (980 °C) Heat treating (or heat treatment) is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the ...
What is isothermal annealing?
Isothermal annealing or process annealing, is slightly different from a full anneal, but produces a similar microstructure. In this process, the part is heated to above the upper critical temperature, and then is cooled quickly to approximately 650°C (1,200°F), and is held isothermally for a period of time.

What is the process of isothermal heat?
Isothermal Process. An isothermal process is a thermodynamic process, in which the temperature of the system remains constant (T = const). The heat transfer into or out of the system typically must happen at such a slow rate in order to continually adjust to the temperature of the reservoir through heat exchange.
Does the temperature change during free expansion?
It is also called Joule expansion. For an ideal gas, the temperature doesn’t change (this means that the process is also isothermal), however, real gases experience a temperature change during free expansion. In free expansion Q = W = 0, and the first law requires that: dEint = 0.
Is pV the same as isothermal gas?
pV = constant. Yes, it seems to be identical as isothermal process of ideal gas. In fact, during their experiments the temperature remain constant as was assumed by Mariotte. These results are fully consistent with ideal gas law, which determinates, that the constant is equal to nRT.
Is adiabatic process an isothermal process?
An adiabatic process is not necessarily an isothermal process, nor is an isothermal process necessarily adiabatic. In engineering, phase changes, such as evaporation or melting, are isothermal processes when, as is usually the case, they occur at constant pressure and temperature.
What is heat treatment?
Heat treating uses strictly controlled temperature modulation to enhance certain desirable characteristics in metals, such as performance and durability. There are five unique heat treatments:
What is isothermal hardening?
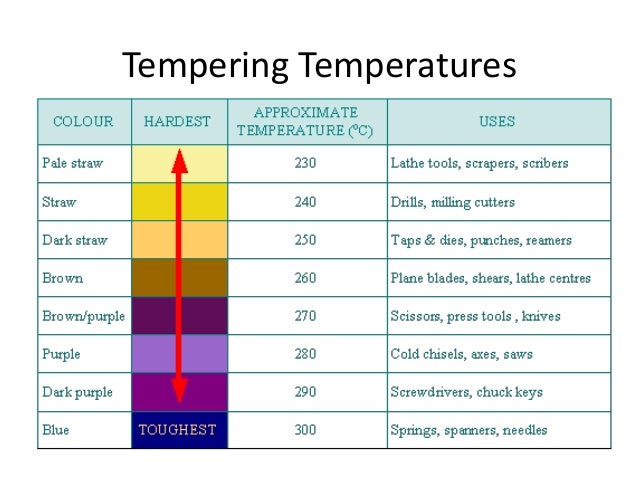
Isothermal hardening is a heat-treating process intended for applications with medium and high carbon-ferrous metals. The primary purpose of isothermal hardening is in reducing distortion while improving the metal’s strength and toughness.
What happens if you heat steel?
If we heat steel, or cast iron until we cause its constituents to go into solution, then quench to 1200°F and hold at that temperature long enough, we will cause that microstructure to transform into the softest microstructure, pearlite. If we had instead cooled that Austenite to 600°F before holding, we would avoid the pearlite transformation and instead create a much harder microstructure, Bainite. Finally, if we cool that workpiece rapidly and sufficiently formation of both pearlite and bainite are bypassed becoming the hardest structure, martensite.
What are the two methods of tempering?
Two well-known tempering methods use Isothermal Hardening; Austempering and Martempering. The two processes share many similarities with their main differences being the temperatures they are being quenched to, the times they remain at those temps, and the end result.
Why is austempered steel used?
Reduced distortion. Austempered steel exhibits reduced distortion due to the quenching process. This makes it particularly useful for thin components that must retain specific dimensions during and after heat treatment.
What does the word "isothermal" mean?
The adjective "isothermal" is derived from the Greek words "ἴσος" ("isos") meaning "equal" and "θέρμη" ("therme") meaning "heat".
How does isothermal compression work?
In the isothermal compression of a gas there is work done on the system to decrease the volume and increase the pressure. Doing work on the gas increases the internal energy and will tend to increase the temperature. To maintain the constant temperature energy must leave the system as heat and enter the environment.
What does the red line on the isothermal expansion line mean?
Black line indicates continuously reversible expansion, while the red line indicates stepwise and nearly reversible expansion at each incremental drop in pressure of 0.1 atm of the working gas.
What is the difference between isothermal and adiabatic processes?
In contrast, an adiabatic process is where a system exchanges no heat with its surroundings ( Q = 0).
Which process is especially convenient for calculating changes in entropy?
Isothermal processes are especially convenient for calculating changes in entropy since, in this case, the formula for the entropy change, Δ S, is simply
Is the free expansion of an ideal gas isothermal?
Once obtained, these formulas can be applied to an irreversible process, such as the free expansion of an ideal gas. Such an expansion is also isothermal and may have the same initial and final states as in the reversible expansion. Since entropy is a state function, the change in entropy of the system is the same as in the reversible process and is given by the formulas above. Note that the result Q = 0 for the free expansion can not be used in the formula for the entropy change since the process is not reversible.
Is the internal energy constant in isothermal processes?
Thus, in an isothermal process the internal energy of an ideal gas is constant. This is a result of the fact that in an ideal gas there are no intermolecular forces. Note that this is true only for ideal gases ; the internal energy depends on pressure as well as on temperature for liquids, solids, and real gases.
What is the name of the institute of technology that treats heat that does not change temperature?
Technion - Israel Institute of Technology. Technically, the answer is obvious: a heat treatment at which the temperature does not change is isothermal, and vise versa.
Is there control over temperature in a furnace?
Usually, there is no control of the sample temperature in a furnace (hence, no information on heating rate is available). The other issue is the duration of exposure of a sample to certain temperature.
How does isothermal annealing work?
In this process, the part is heated to above the upper critical temperature, and then is cooled quickly to approximately 650°C (1,200°F), and is held isothermally for a period of time. The austenite transforms to ferrite and pearlite. The part is then withdrawn from the furnace, and allowed to air cool in some convenient manner. The advantage of an isothermal anneal over a process anneal is predominately shorter time. A full anneal will require about 30 hours, but an isothermal anneal will require approximately four hours, depending on the alloy. This is shown in Figure 3.
What temperature is normalizing?
Normalizing is a similar process to full annealing, but with some important differences. When normalizing, the temperatures are approximately 25°C above the normal hardening or austenitizing temperature. After complete transformation to austenite (generally soaked at temperature for one hour per inch or 25 mm of thickness), the part is withdrawn from the furnace and allowed to air cool. These processes are typically performed on weldments, forgings or castings.
How is spheroidized steel annealed?
In subcritical annealing, the steels are heated to just below the A1 temperature and held for an extended period of time (usually many hours). The steels are then cooled to room temperature in some convenient manner (usually air cooling). The parts do not transform to austenite, and so it is possible that some elements of the prior microstructure can remain. A fine pearlitic structure could be maintained to decrease the diffusion distance and improve kinetics. The structures of subcritical spherodized parts usually contain fine spherical cementite inside ferrite grains. The final carbide size is adjusted with the selected heat treatment time and temperature.
What is homogenization annealing?
Homogenization Annealing is an annealing method that is used at the steel mill. This annealing process is somewhat specialized, in that the purpose is to level out segregation in steel ingots or continuously cast strip. Very high temperatures and very long times are used to allow variations in chemistry due to segregation to level out. This segregation is why there is a different hardenability at the ends of a coil. Once ready to be cooled, the ingots or coils are removed from the furnace, and allowed to air-cool. Because air cooling is relatively uncontrolled, variations in grain size and microstructure can occur. This often explains the different performance of one mill over the other during forming, even while meeting the procurement specifications.
Why is annealing used in steel?
But in all cases, the primary reason for annealing is to soften the part and increase the ductility for forming or machining. Figure 1: Typical homogenization anneal of ingots in a soaking pit at a steel mill. Homogenization Annealing is an annealing method that is used at the steel mill.
Why do we spheroize steel?
The primary reason for spheroidizing is to produce a very ductile steel suitable for deep forming, or forming in complex shapes. The spherical carbides allow the steel to plastically deform without cracking. It also reduces die wear by reducing the necessary pressure for forming. Since long furnace times are necessary to produce the spheroidal carbides, this annealing practice is usually reserved for difficult to form parts, or where the extended die life can justify the increased costs of spheroidizing.
What is the difference between normalizing and annealing?
The largest difference between full annealing and normalizing, is the lamellar spacing of the resulting pearlite. Very coarse pearlite is very soft. Fine grained pearlite is harder, and somewhat easier to machine. There is also less work hardening during machining, because the steel is less “gummy.”.
How does heat treatment work?
These tend to consist of either cooling different areas of an alloy at different rates, by quickly heating in a localized area and then quenching, by thermochemical diffusion, or by tempering different areas of an object at different temperatures, such as in differential tempering.
What is the purpose of heat treating metals?
grain size and composition) is one of the most effective factors that can determine the overall mechanical behavior of the metal. Heat treatment provides an efficient way to manipulate the properties of the metal by controlling the rate of diffusion and the rate of cooling within the microstructure. Heat treating is often used to alter the mechanical properties of a metallic alloy, manipulating properties such as the hardness, strength, toughness, ductility, and elasticity .
What is differential hardening?
Some techniques allow different areas of a single object to receive different heat treatments. This is called differential hardening. It is common in high quality knives and swords. The Chinese jian is one of the earliest known examples of this, and the Japanese katana may be the most widely known. The Nepalese Khukuri is another example. This technique uses an insulating layer, like layers of clay, to cover the areas that are to remain soft. The areas to be hardened are left exposed, allowing only certain parts of the steel to fully harden when quenched.
How does salt heat up?
Parts are loaded into a pot of molten salt where they are heated by conduction, giving a very readily available source of heat. The core temperature of a part rises in temperature at approximately the same rate as its surface in a salt bath.
What is a heat treating schedule?
Complex heat treating schedules, or " cycles," are often devised by metallurgists to optimize an alloy's mechanical properties. In the aerospace industry, a superalloy may undergo five or more different heat treating operations to develop the desired properties. This can lead to quality problems depending on the accuracy of the furnace's temperature controls and timer. These operations can usually be divided into several basic techniques.
What is the process of heating something to alter it?
Process of heating something to alter it. Heat treating furnace at 1,800 °F (980 °C) Heat treating (or heat treatment) is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the ...
Why is heat treatment called an arrest?
This temperature is referred to as an "arrest" because at the A temperature the metal experiences a period of hysteresis.
Overview
In thermodynamics, an isothermal process is a type of thermodynamic process in which the temperature T of a system remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir, and a change in the system occurs slowly enough to allow the system to be continuously adjusted to the temperature of the reservoir through heat exchang…
Etymology
The adjective "isothermal" is derived from the Greek words "ἴσος" ("isos") meaning "equal" and "θέρμη" ("therme") meaning "heat".
Examples
Isothermal processes can occur in any kind of system that has some means of regulating the temperature, including highly structured machines, and even living cells. Some parts of the cycles of some heat engines are carried out isothermally (for example, in the Carnot cycle). In the thermodynamic analysis of chemical reactions, it is usual to first analyze what happens under isothermal conditions and then consider the effect of temperature. Phase changes, such as melti…
Details for an ideal gas
For the special case of a gas to which Boyle's law applies, the product pV (p for gas pressure and V for gas volume) is a constant if the gas is kept at isothermal conditions. The value of the constant is nRT, where n is the number of moles of the present gas and R is the ideal gas constant. In other words, the ideal gas law pV = nRT applies. Therefore:
Calculation of work
In thermodynamics, the reversible work involved when a gas changes from state A to state B is
where p for gas pressure and V for gas volume. For an isothermal (constant temperature T), reversible process, this integral equals the area under the relevant PV (pressure-volume) isotherm, and is indicated in purple in Figure 2 f…
Example of an isothermal process
The reversible expansion of an ideal gas can be used as an example of work produced by an isothermal process. Of particular interest is the extent to which heat is converted to usable work, and the relationship between the confining force and the extent of expansion.
During isothermal expansion of an ideal gas, both p and V change along an iso…
Entropy changes
Isothermal processes are especially convenient for calculating changes in entropy since, in this case, the formula for the entropy change, ΔS, is simply
where Qrev is the heat transferred (internally reversible) to the system and T is absolute temperature. This formula is valid only for a hypothetical reversible process; that is, a process in which equilibrium is maintained at all times.
See also
• Joule–Thomson effect
• Joule expansion (also called free expansion)
• Adiabatic process
• Cyclic process