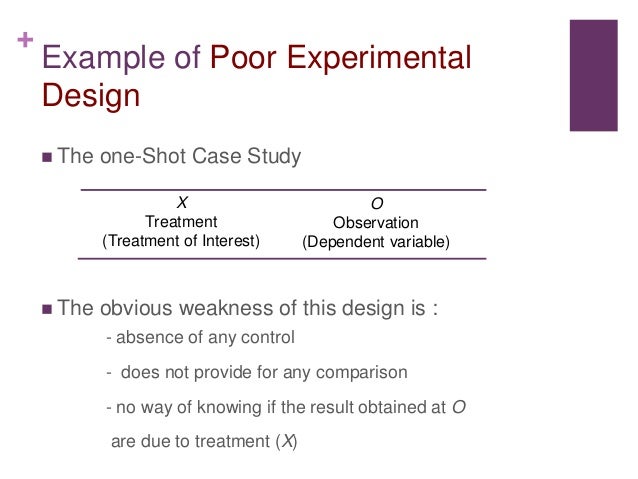
Basic concepts
- Treatment and control groups. In experimental research, some subjects are administered one or more experimental stimulus called a treatment (the treatment group) while other subjects are not given such a ...
- Treatment manipulation. ...
- Random selection and assignment. ...
- Threats to internal validity. ...
What does experimental treatment mean?
· Experimental research is considered a standard method that uses observations, simulations and surveys to collect data. One of its unique features is the ability to control extraneous variables and their effects. It’s a suitable method for those looking to examine the relationship between cause and effect in a field setting or in a laboratory.
What is a treatment in an experiment?
· Clinical trials are experiments designed to determine if a new medication or treatment is safe and effective in humans. The foundation of any clinical trial is the comparison between 2 groups of participants—usually one group that is receiving a type of intervention and another group that is receiving a different intervention or no intervention.
What is the treatment of an experiment?
Experimental Treatments and Clinical Trials Summary Most health plans don’t cover treatments they regard as “experimental.” Sometimes, they may deny a claim for such a treatment. But, you and your doctor may think the treatment is well supported by evidence. Then, you have grounds for an appeal. Appealing a decision.
What is the purpose of experimental research?
In true experimental research, the researcher not only manipulates the independent variable, he or she also randomly assigned individuals to the various treatment categories (i.e., control and treatment). In quasi experimental research, the researcher does not randomly assign subjects to treatment and control groups. In other words, the treatment is not distributed among …
What is an example of experimental treatment?
and the “treatment” is the variable you are studying. For example, a human experimental group could receive a new medication, a different form of counseling, or some vitamin supplements. A plant treatment group could receive a new plant fertilizer, more sunlight, or distilled water.
How do you identify experimental treatment?
Treatments are administered to experimental units by 'level', where level implies amount or magnitude. For example, if the experimental units were given 5mg, 10mg, 15mg of a medication, those amounts would be three levels of the treatment.
What is an example of experimental research?
Experimental research examples are different, depending on the type of experimental research design that is being considered. The most basic example of experimental research is laboratory experiments, which may differ in nature depending on the subject of research.
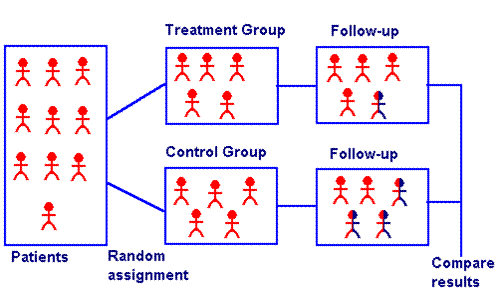
What is an experimental treatment group?
The treatment group (also called the experimental group) receives the treatment whose effect the researcher is interested in. The control group receives either no treatment, a standard treatment whose effect is already known, or a placebo (a fake treatment).
What is the purpose of treatment in research?
Treatment groups are the sets of participants in a research study that are exposed to some manipulation or intentional change in the independent variable of interest. They are an integral part of experimental research design that helps to measure effects as well as establish causality.
What are the 3 characteristics of experimental research?
Several kinds of experimental designs exist. In general, designs that are true experiments contain three key features: independent and dependent variables, pretesting and posttesting, and experimental and control groups. In a true experiment, the effect of an intervention is tested by comparing two groups.
What are some examples of experiment?
An example of an experiment is when scientists give rats a new medicine and see how they react to learn about the medicine. An example of an experiment is when you try a new coffee shop but you aren't sure how the coffee will taste. The process of conducting such a test; experimentation.
Is experimental research qualitative or quantitative?
QuantitativeQuantitative designs can be experimental, quasi-experimental, descriptive, or correlational. Qualitative is usually more subjective, although like quantitative research, it also uses a systematic approach. Qualitative research is generally preferred when the clinical question centers around life experiences or meaning.
How to find experimental treatment?
You can also search the hospital website or the website of nearby universities by searching for 'clinical trials,' or 're search.'
Who funds experimental research?
Pharmaceutical companies, medical device manufacturers, government grants, foundations or non-profit charities often fund the experimental costs. Sometimes, research scientists at universities receive funding from one or more of these sources and may work in collaboration with teams from multiple clinics.
What is the foundation of clinical trials?
The foundation of any clinical trial is the comparison between 2 groups of participants — usually one group who is receiving a type of intervention and another group who is receiving a different intervention or no intervention.
What is the approval process for clinical trials?
Approval and oversight of clinical trials are quite stringent — requiring detailed applications and approvals at multiple levels. Researchers must be experienced and qualified in order to obtain authorization to implement clinical trials. Generally, a hospital or university or pharmaceutical manufacturer requires preliminary data on safety, sometimes obtained through animal testing, before allowing a human study. Usually, a federal organization, such as the Federal Food and Drug Administration provides structured oversight and criteria.
What does "the best treatment" mean?
For some, the best treatment means the safest and most proven available. For others, the best intervention means the absolute best around — anywhere — even if it hasn't been tried and true or proven to be safe.
Does it hurt to learn about experimental treatment?
No matter where you see yourself on the spectrum, it never hurts to learn about an experimental treatment for your illness, and learning about them does not require you to sign up.
Can a doctor suggest a clinical trial?
In some cases, your doctor might suggest a clinical trial for you to give you access to the treatment you might not otherwise be able to get. You can ask your doctor if there is an experimental treatment that you qualify for.
What is clinical trial?
Clinical trials. In other cases, an experimental, or investigational, treatment may be just what you want. For example, you may have tried standard treatments without success. You may want to take part in a clinical trial—a study in humans—of a promising new treatment. To start looking for such a trial, ask your doctor.
What to do if you can't enroll in a clinical trial?
What to do if you can’t enroll in a clinical trial but still want an investigational treatment. Appealing Decisions That a Treatment Is Experimental. If your insurer denies your claim because it says the treatment is experimental, follow your insurer’s appeals process. Also, follow the advice in Appealing a Reimbursement Decision.
How to look for a trial?
The first place to start looking for such a trial is your doctor. Doctors often know about trials that might help you. They may be able to search for one for you. They can also talk over with you the pros and cons of enrolling in a trial. If your regular doctor doesn’t help, consider seeking a second opinion.
Do you pay for routine care in a trial?
But, often, the trial sponsor will supply that treatment for free. Usually, you’ll keep on getting routine care from your own doctor, and your insurer should continue to pay for that. In the trial setting, most plans are required to pay for routine care costs under certain conditions. Expanded Access.
Do health insurance plans cover experimental treatments?
Most health plans don’t cover treatments they regard as “experimental.” Sometimes, they may deny a claim for such a treatment. But, you and your doctor may think the treatment is well supported by evidence. Then, you have grounds for an appeal.
Does insurance pay for trial?
Expect your insurer to pay for routine care costs linked to the trial, assuming certain conditions are met. Don’t expect the insurer to pay for the investigational treatment itself or for research costs .
What distinguishes experimental research from other types of research?
The major feature that distinguishes experimental research from other types of research is that the researcher manipulates the independent variable. There are a number of experimental group designs in experimental research. Some of these qualify as experimental research, others do not.
What is experimental treatment diffusion?
Experimental Treatment Diffusion — Sometimes the control group actually implements the treatment. If two different techniques are being tested in two different third grades in the same building, the teachers may share what they are doing. Unconsciously, the control may use of the techniques she or he learned from the treatment teacher.
How does testing affect the results of an experiment?
Testing — The act of taking a pretest or posttest may influence the results of the experiment. Suppose we were conducting a unit to increase student sensitivity to prejudice. As a pretest we have the control and treatment groups watch Shindler’s List and write a reaction essay. The pretest may have actually increased both groups’ sensitivity and we find that our treatment groups didn’t score any higher on a posttest given later than the control group did. If we hadn’t given the pretest, we might have seen differences in the groups at the end of the study.
What is quasi experimental research?
In this case, quasi-experimental research involves using intact groups in an experiment, rather than assigning individuals at random to research conditions. (some researchers define this latter situation differently.
Why does causal comparative research not meet the standards of an experiment?
It does not meet the standards of an experiment because the independent variable in not manipulated. The statistics by themselves have no meaning. They only take on meaning within the design of your study.
What is loss of subjects?
Loss of Subjects (Mortality) — All of the high or low scoring subject may have dropped out or were missing from one of the groups. If we collected posttest data on a day when the honor society was on field trip at the treatment school, the mean for the treatment group would probably be much lower than it really should have been.
What is experimental research?
Experimental research is a quantitative research method with a scientific approach, where a set of variables are kept constant while the other set of variables are being measured as the subject of an experiment. Learn about various types of experimental research design along with its advantages. Products .
Why is experimental research important?
Experimental research allows you to test your idea in a controlled environment before taking it to market . It also provides the best method to test your theory, thanks to the following advantages: Researchers have a stronger hold over variables to obtain desired results.
What is quasi research?
Quasi-research is used in field settings where random assignment is either irrelevant or not required. Learn about: Market research. Advantages of experimental research.
What is a quasi-experimental design?
3. Quasi-experimental research design: The word “Quasi” indicates similarity. A quasi-experimental design is similar to experimental, but it is not the same. The difference between the two is the assignment of a control group. In this research, an independent variable is manipulated, but the participants of a group are not randomly assigned. Quasi-research is used in field settings where random assignment is either irrelevant or not required.
How many types of experimental design are there?
There are three primary types of experimental design:
What is the difference between experimental and quantitative research?
The first set acts as a constant, which you use to measure the differences of the second set. Quantitative research methods, for example, are experimental. If you don’t have enough data to support your decisions, you must first determine the facts. Experimental research gathers the data necessary to help you make better decisions.
Why is time important in research?
Time is a vital factor in establishing a relationship between cause and effect. Invariable behavior between cause and effect. You wish to understand the importance of the cause and effect. Learn about: Quantitative Market Research. Types of experimental research design.
What is experimental research?
Experimental research is the most familiar type of research design for individuals in the physical sciences and a host of other fields. This is mainly because experimental research is a classical scientific experiment, similar to those performed in high school science classes.
What are the three types of experimental research?
They are of 3 types, namely; pre-experimental, quasi-experimental, and true experimental research.
What is the difference between independent and extraneous variables?
The independent variables are the experimental treatment being exerted on the dependent variables. Extraneous variables , on the other hand , are other factors affecting the experiment that may also contribute to the change.
Why is it so difficult to conclude a non-experimental study?
This is because it takes place in a real-life setting, where extraneous variables cannot be eliminated. Therefore, it is more difficult to conclude non-experimental studies, even though they are much more flexible and allow for a greater range of study fields.
What is a posttest study?
The study is carried out after some treatment which was presumed to cause change, making it a posttest study.
What is pre experimental design?
In pre-experimental research design, either a group or various dependent groups are observed for the effect of the application of an independent variable which is presumed to cause change. It is the simplest form of experimental research design and is treated with no control group.
What is a quasi experiment?
In quasi-experiments, the participants are not randomly assigned, and as such, they are used in setting s where randomization is difficult or impossible.
What is experimental research?
In experimental research, the researcher manipulates the independent or treatment variable (s) and then observes whether the treatment groups differ on one or more dependent or outcome variables. In multiple-case research, the scores of two or more groups of cases (which might be the same research units or might be ...
What is multiple case research?
In multiple-case research, the scores of two or more groups of cases (which might be the same research units or might be ...
What is clinical research?
Clinical research is much different from the medical treatment you receive in a Healthcare Provider's office. Answers specific questions through research involving numerous research volunteers. Address the needs of individual patients.
Why do people participate in clinical trials?
Some people participate in clinical trials because none of the standard (approved) treatment options have worked, or they are unable to tolerate certain side effects. Clinical trials provide another option when standard therapy has failed.
How does the FDA work?
FDA works to protect participants in clinical trials and to ensure that people have reliable information before deciding whether to join a clinical trial. The Federal government has regulations and guidelines for clinical research to protect participants from unreasonable risks.
What does the FDA do?
FDA seeks to ensure that people of different ages, races, ethnic groups, and genders are included in clinical trials. Learn more about FDA’s efforts to increase diversity in clinical trials.
Why is confidentiality important in clinical research?
Confidentiality is an important part of clinical research and ensures that personal information is seen only by those authorized to have access. It also means that the personal identity and all medical information of clinical trial participants is known only to the individual patient and researchers.
How to find out if there are clinical trials?
One good way to find out if there are any clinical trials that might help you is to ask your doctor. Other sources of information include:
What are the criteria for clinical trials?
All clinical trials have guidelines, called eligibility criteria, about who can participate. The criteria are based on such factors as age, sex, type and stage of disease, previous treatment history, and other medical conditions.
