
Medication
Mar 17, 2011 · There is no cure or effective treatment for Tay-Sachs disease. However, researchers are pursuing several approaches to finding a cure. Scientists are exploring enzyme replacement therapy to provide the Hex-A that is lacking in babies with Tay-Sachs.
Therapy
Sep 20, 2016 · A few different therapies are being explored as potential treatments for Tay-Sachs disease. These include gene therapy, stem cell therapy, enzyme replacement therapy, substrate inhibition, and pharmalogical chaperone therapy. Gene therapy replaces the defective gene in a person with an unaffected one that can produce the necessary ...
Self-care
Nutrition
See more
What is the life expectancy of someone with Tay Sachs?
Is there cure for Tay Sachs?
What are the chances of having Tay Sachs disease?
What are treatments for Tay Sachs disease?
What type of help is available for a child with Tay-Sachs disease?
Genetic testing is available for couples who may face a higher risk for having a baby with Tay-Sachs. Genetic testing and counseling can help parents-to-be make informed decisions about family planning.Dec 18, 2020
Is gene therapy an Option for Tay-Sachs?
Gene Therapy Approach Gene therapy is being researched to potentially treat both Tay-Sachs and Sandhoff disease. Gene therapy aims to be a one-time treatment that could slow or stop disease progression by delivering working HEXA and HEXB genes into the cells using a viral vector.Sep 28, 2021
What are two examples of promising future strategies for treating the disease mechanisms that lead to Tay-Sachs?
Potential treatments include:Enzyme replacement therapy. Since Tay-Sachs is caused by the lack of the Hex-A enzyme, this treatment seeks to replace the enzyme. ... Enzyme-enhancing therapy. ... Substrate reduction therapy. ... Gene therapy. ... Cell transplantation.
Is Tay-Sachs dominant or recessive?
Inheritance. This condition is inherited in an autosomal recessive pattern , which means both copies of the gene in each cell have variants. The parents of an individual with an autosomal recessive condition each carry one copy of the altered gene, but they do not show signs and symptoms of the condition.Sep 30, 2021
Is Tay-Sachs autosomal or Sexlinked?
No. Tay-Sachs disease is an autosomal recessive condition. Sex-linked conditions are caused by genes located on a sex chromosome (X or Y). Tay-Sachs disease is caused by a gene (HEXA) located on chromosome 15, an autosome .Mar 30, 2015
What is Tay-Sachs enzyme replacement?
ERT. ERT has been considered as an approach for the treatment of Tay-Sachs disease and other lysosomal storage disorders. The aim of ERT is to provide a replacement of the HEXA enzyme that can play the role of the non-functional enzyme of individuals with the disease.May 17, 2021
How much does it cost to treat Tay-Sachs?
Peter (1975) states that the cost of caring for a Tay-Sachs baby is estimated to be between $30,000 to $40,000 per year.
What protein is affected by Tay-Sachs?
Tay-Sachs disease occurs when the body lacks hexosaminidase A. This is a protein that helps break down a group of chemicals found in nerve tissue called gangliosides. Without this protein, gangliosides, particularly ganglioside GM2, build up in cells, often nerve cells in the brain.Oct 27, 2020
What is Tay-Sachs disease?
Tay-Sachs disease belongs to the group of autosomal-recessive lysosomal storage metabolic disorders. This disease is caused by β-hexosaminidase A (HexA) enzyme deficiency due to various mutations in α-subunit gene of this enzyme, resulting in GM2 ganglioside accumulation predominantly in lysosomes of nerve cells. Tay-Sachs disease is characterized by acute neurodegeneration preceded by activated microglia expansion, macrophage and astrocyte activation along with inflammatory mediator production. In most cases, the disease manifests itself during infancy, the “infantile form,” which characterizes the most severe disorders of the nervous system. The juvenile form, the symptoms of which appear in adolescence, and the most rare form with late onset of symptoms in adulthood are also described. The typical features of Tay-Sachs disease are muscle weakness, ataxia, speech, and mental disorders. Clinical symptom severity depends on residual HexA enzymatic activity associated with some mutations. Currently, Tay-Sachs disease treatment is based on symptom relief and, in case of the late-onset form, on the delay of progression. There are also clinical reports of substrate reduction therapy using miglustat and bone marrow or hematopoietic stem cell transplantation. At the development stage there are methods of Tay-Sachs disease gene therapy using adeno- or adeno-associated viruses as vectors for the delivery of cDNA encoding α and β HexA subunit genes. Effectiveness of this approach is evaluated in α or β HexA subunit defective model mice or Jacob sheep, in which Tay-Sachs disease arises spontaneously and is characterized by the same pathological features as in humans. This review discusses the possibilities of new therapeutic strategies in Tay-Sachs disease therapy aimed at preventing neurodegeneration and neuroinflammation.
What is SRT in a TSD?
Substrate reduction therapy (SRT) utilizes small molecules to slow the rate of glycolipid biosynthesis ( Platt et al., 2003 ). Efficacy of miglustat (N-butyldeoxynojirimycin, NB-DNJ) in the prevention of GM2 ganglioside accumulation was demonstrated in TSD murine models ( Platt et al., 1997; Bembi et al., 2006 ). NB-DNJ is a small iminosugar competitive inhibitor of glucosylceramide synthase. It catalyzes the first committed step of glycosphingolipid synthesis and can penetrate the blood-brain barrier ( Boomkamp et al., 2010 ). It has been shown that that NB-DNJ added to the food of TSD model mice reduces GM2 ganglioside accumulation in the brain by 50% ( Platt et al., 1997 ). Bembi et al. (2006) assessed the clinical efficacy of NB-DNJ in two patients with TSD infantile form. The authors observed a significant concentration of NB-DNJ in the cerebrospinal fluid of the patients. The use of SRT did not stop the neurologic dysfunction progression in patients, however, the authors recommend the use of NB-DNJ for macrocephaly prevention ( Bembi et al., 2006 ). Similar results were described in a clinical trial (#N#{"type":"clinical-trial","attrs": {"text":"NCT00672022","term_id":"NCT00672022"}}#N#NCT00672022) in five patients ( Maegawa et al., 2009 ).
What is GM2 gangliosidose?
GM2-gangliosidoses are a group of autosomal-recessive lysosomal storage disorders (LSDs). These diseases result from a deficiency of lysosomal enzyme β-hexosaminidase (Hex), which is responsible for GM2 ganglioside degradation ( Ferreira and Gahl, 2017 ). Gangliosides are the main glycolipids of neuronal cell plasma membranes which ensure normal cellular activities ( Sandhoff and Harzer, 2013 ). There are two major β-hexosaminidase isoenzymes: HexA consists of two subunits, α and β; HexB is a homodimer consisting of two β-subunits ( Ferreira and Gahl, 2017 ). The two subunits of HexA enzyme, α and β, are synthesized at the endoplasmic reticulum (ER) where glycosylation, the formation of intramolecular disulfide bonds and dimerization take place ( Weitz and Proia, 1992; Maier et al., 2003 ). Besides HexA and HexB isoenzymes a homodimer consisting of two α-subunits, called HexS, is also found ( Hou et al., 1996 ).
What are the causes of GM2 gangliosis?
GM2-gangliosidosis can be caused by mutations in three genes: HEXA (15th chromosome), HEXB (5th chromosome), and GM2A (5th chromosome) ( Mahuran, 1999; Ferreira and Gahl, 2017 ). GM2-gangliosidosis includes (I) Tay-Sachs disease (TSD, OMIM 272800), at which mutations occur in the HEXA gene and only HexA activity is disrupted (variant B); (II) Sandhoff disease (SD; OMIM 268800), caused by mutations in HEXB gene, at which the activity of HexA and HexB is disrupted (variant O); and (III) GM2 activator protein deficiency (OMIM 272750), at which mutations take place in the GM2A gene (variant AB) ( Mahuran, 1999 ).
What are the glycolipids of the plasma membrane?
Gangliosides are the main glycolipids of neuronal cell plasma membranes which ensure normal cellular activities ( Sandhoff and Harzer, 2013 ). There are two major β-hexosaminidase isoenzymes: HexA consists of two subunits, α and β; HexB is a homodimer consisting of two β-subunits ( Ferreira and Gahl, 2017 ).
What is the hexa level in leukocytes?
HexA activity in leukocytes was 187 nmol/mg/h, which is comparable to the enzyme activity in control group leukocytes. HexA activity in plasma was 15 nmol/mg/h, which is three times lower than the lower limit of HexA normal activity (50–250 nmol/mg/h).
When does adolescence manifest?
In most cases, the disease manifests itself during infancy, the “infantile form,” which characterizes the most severe disorders of the nervous system. The juvenile form, the symptoms of which appear in adolescence, and the most rare form with late onset of symptoms in adulthood are also described.
What happens when GM2 ganglioside is toxic?
As a result, this substance accumulates to toxic levels, particularly in neurons in the brain and spinal cord. Progressive damage caused by the buildup of GM2 ganglioside leads to the destruction of these neurons, which causes the signs and symptoms seen in Tay-Sachs disease. [3] Last updated: 3/30/2015.
What is Tay-Sachs disease?
Tay-Sachs disease is caused by mutations in the HEXA gene and inheritance is autosomal recessive. The HEXA gene gives the body instructions to make part of the beta-hexosaminidase A enzyme, which is needed to break down a substance called GM2 ganglioside. When the enzyme is not functional or not made, GM2 ganglioside builds up in the nerve cells ...
What is the HPO database?
People with the same disease may not have all the symptoms listed. This information comes from a database called the Human Phenotype Ontology (HPO) . The HPO collects information on symptoms that have been described in medical resources.
What is the Lysosomal Disease Network?
The Lysosomal Disease Network is a team of doctors, nurses, research coordinators, and research labs throughout the U.S., working together to improve the lives of people with this condition through research. The Lysosomal Disease Network has a registry for patients who wish to be contacted about clinical research opportunities.
Where does GM2 build up?
When the enzyme is not functional or not made, GM2 ganglioside builds up in the nerve cells (neurons) of the brain and spinal cord , causing the symptoms of the disease. [3] The diagnosis of Tay-Sachs disease involves a blood test that detects absent or very low levels of beta-hexosaminidase A enzyme activity.
What is GHR in MedlinePlus?
MedlinePlus was designed by the National Library of Medicine to help you research your health questions, and it provides more information about this topic. Genetics Home Reference (GHR) contains information on Tay-Sachs disease. This website is maintained by the National Library of Medicine.
What are the symptoms of neuropathy?
Neurological impairment is slowly progressive and may lead to clumsiness and loss of coordination, muscle weakness, tremors, difficulty speaking or swallowing, and uncontrollable muscle spasms and movements. Many people eventually need mobility assistance.
What causes tay sachs in babies?
Babies with Tay-Sachs lack a particular enzyme, which is a protein that triggers chemical reactions in cells. The lack of the enzyme, hexosaminidase A, causes a fatty substance to collect. The buildup of this substance, GM2 ganglioside, leads to Tay-Sachs symptoms such as muscle weakness. Tay-Sachs is a genetic condition.
What is tay sachs disease?
Tay-Sachs disease is a genetic condition. Tay-Sachs is caused by a baby receiving two defective HEXA genes, one from each parent. Tay-Sachs disease symptoms include failing to meet motor milestones, such as sitting and standing. Babies born with Tay-Sachs often die at a young age. Genetic testing can help you make family planning decisions.
How does tay sachs happen?
Tay-Sachs happens when both parents had a variant HEXA gene and passed it on. That means neither copy of the baby’s HEXA gene works well.
How does Tay-Sachs disease affect children?
It’s more common among people from certain backgrounds, such as Ashkenazi Jewish people. Tay-Sachs disease symptoms include muscle weakness. Children also miss motor milestones, such as not pulling up to standing. There’s no cure for Tay-Sachs disease. Treatment seeks to improve quality of life and keep children comfortable. Most children born with Tay-Sachs pass away before their fifth birthday. Genetic testing can help you find out if you or your partner is a Tay-Sachs carrier. You can then make informed decisions about family planning. If you’re planning a pregnancy and at high risk for Tay-Sachs, talk to your healthcare provider.
What ethnicity is affected by Tay-Sachs disease?
People across racial and ethnic groups can carry a genetic change tied to Tay-Sachs disease. But it’s much more common among people of Ashkenazi (Eastern European) Jewish descent. Other populations with higher numbers of people carrying the disease-causing genetic change include: French Canadians.
How many people carry the variant gene?
For people not from high-risk backgrounds, around 1 in 300 people carry the genetic change (or variant gene) for Tay-Sachs. For people of Ashkenazi Jewish descent: About 1 in 30 people carry the variant gene. About 1 in 3,600 newborns is affected.
How long does it take for a child to develop tay sachs?
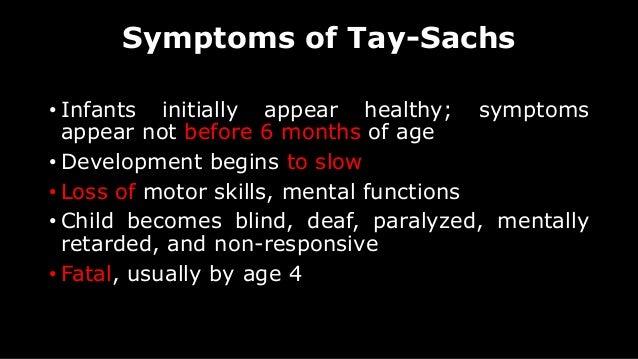
Children develop symptoms around 6 months.
What is tay sachs disease?
What Do We Know About Heredity and Tay-Sachs Disease? Tay-Sachs disease (TSD) is a fatal genetic disorder, most commonly occurring in children, that results in progressive destruction of the nervous system.
What is the chance of a child inheriting a defective gene?
Carriers have a 50 percent chance of passing on the defective gene to their children. A child who inherits one inactive gene is a Tay-Sachs carrier like the parent. If both parents are carriers and their child inherits the defective Hex-A gene from each of them, the child will have Tay-Sachs disease.
What is the cause of Tay-Sachs?
Tay-Sachs is caused by the absence of a vital enzyme called hexosaminidase-A (Hex-A). Without Hex-A, a fatty substance, or lipid, called GM2 ganglioside accumulates abnormally in cells, especially in the nerve cells of the brain.
What is enzyme assay?
The enzyme assay is a biochemical test that measures the level of Hex-A in a person's blood. Carriers have less Hex-A in their body fluid and cells than non-carriers. DNA-based carrier testing looks for specific mutations or changes in the gene that codes for Hex-A.
How to identify Tay-Sachs carriers?
A simple blood test can identify Tay-Sachs carriers. Blood samples can be analyzed by either enzyme assay or DNA studies. The enzyme assay is a biochemical test that measures the level of Hex-A in a person's blood. Carriers have less Hex-A in their body fluid and cells than non-carriers.
When can a fetus be tested for tay sachs?
Alternatively, the fetus can be tested with amniocentesis around the 16th week of pregnancy. In this procedure, a needle is used to remove and test a sample of the fluid surrounding the baby. Assisted reproductive therapy is an option for carrier couples who don't want to risk giving birth to a child with Tay-Sachs.
How old is a baby with Tay-Sachs?
However, a baby with Tay-Sachs disease appears normal until about six months of age when its development slows. By about two years of age, most children experience recurrent seizures and diminishing mental function. The infant gradually regresses, and is eventually unable to crawl, turn over, sit or reach out.
Treatment
After a diagnosis of Tay-Sachs disease is confirmed, families should seek a medical consultation with a physician such as a metabolic genetic specialist with experience in the disorder.
Share Your Experiences
Please consider sharing your experience on social media to help your friends and family start their genetic journeys.
Overview
Genetics
Epidemiology
Symptoms
Specialist to consult
Prognosis
Types
- There is no cure for Tay-Sachs disease, and no treatments are currently proved to slow progression of the disease. Some treatments can help in managing symptoms and preventing complications. The goal of treatment is support and comfort. Supportive treatments include: 1. Medication.A number of prescription medications are available to reduce symptom...