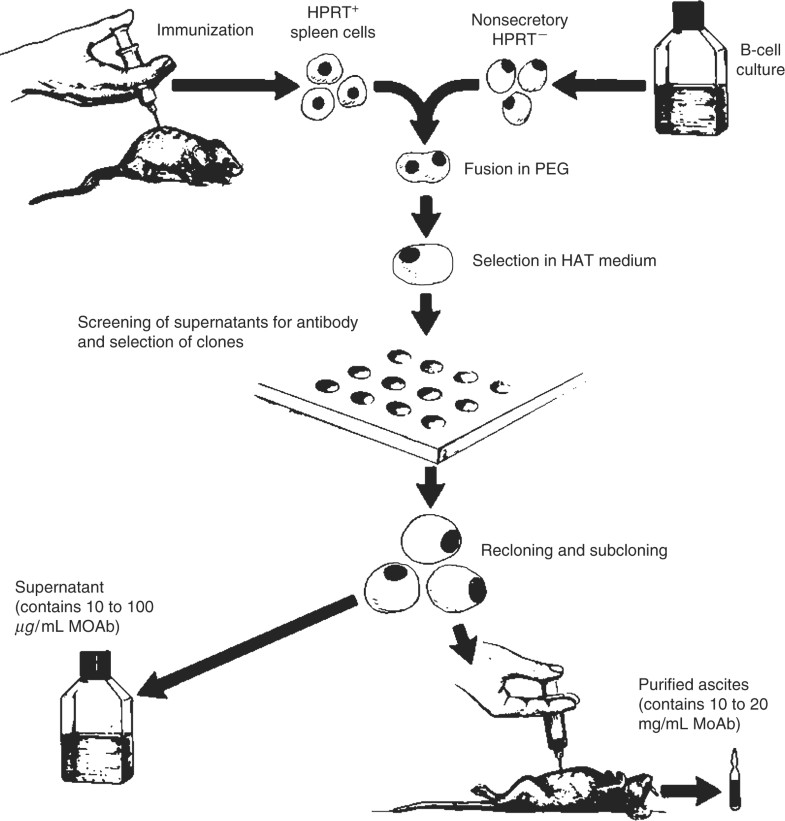
The initial monoclonal antibodies
Monoclonal antibody
Monoclonal antibodies are antibodies that are made by identical immune cells that are all clones of a unique parent cell. Monoclonal antibodies can have monovalent affinity, in that they bind to the same epitope. In contrast, polyclonal antibodies bind to multiple epitopes and are usually made b…
Full Answer
What do you know about monoclonal antibody therapy?
Sep 11, 2014 · 2. Generation of monoclonal antibodies using the hybridoma technique. Monoclonal antibodies are monovalent antibodies which bind to the same epitope and are produced from a single B-lymphocyte clone . They were first generated in mice in 1975 using a hybridoma technique . The generation of hybridomas involves immunising a certain species …
How effective is the monoclonal treatment?
Nov 29, 2021 · Monoclonal antibodies are typically derived from a clonal expansion of antibody producing malignant human plasma cells. The initial monoclonal antibodies were created by fusing spleen cells from an immunized mouse with human or mouse myeloma cells (malignant self-perpetuating antibody producing cells), and selecting out and cloning the hybrid cells …
Who pays for monoclonal treatment?
Apr 10, 2021 · Therapeutic monoclonal antibodies have been applied to treat human diseases since 1986 when muromonab-CD3 was approved by FDA for treating acute rejection after a kidney transplant 60. It has rapidly become a major part of the pharmaceutical industry for the past 35 years. At present, its annual market reaches 150 billion US dollars.
What are the dangers of monoclonal antibodies?
close contact with someone who has tested positive, you may want to consider a monoclonal antibody (mAb) treatment. You may qualify for a mAb treatment ... but this can take weeks to develop enough antibodies against a virus. So, if you have the virus, the mAb treatment gives your body the antibodies it needs to protect ...
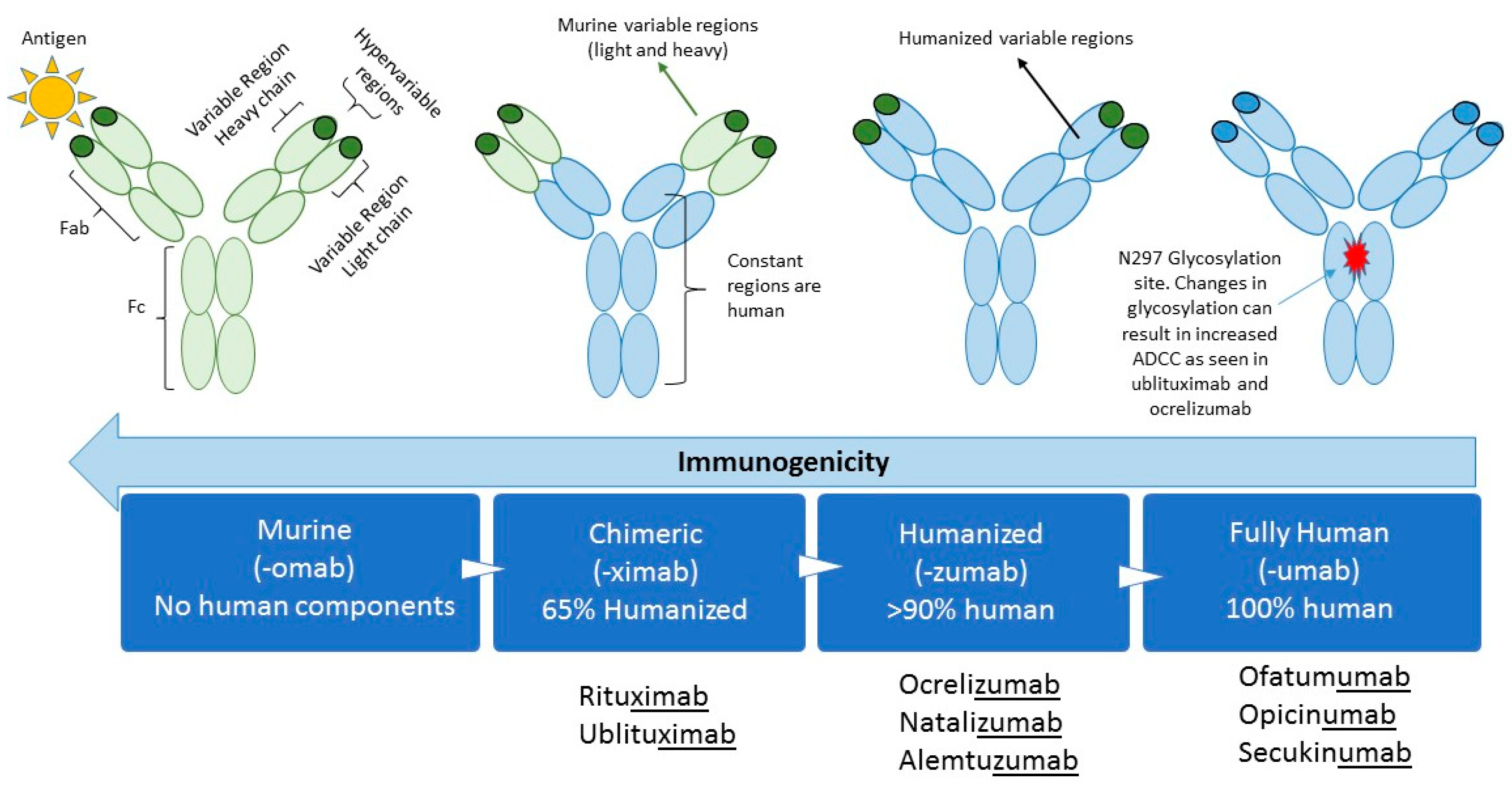
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
Can I get the COVID-19 vaccine if I was treated with monoclonal antibodies or convalescent plasma?
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
How do monoclonal antibodies work against COVID-19?
Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
Should you still get the COVID-19 vaccine if you were treated with monoclonal antibodies?
If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, there is no need to delay getting a COVID-19 vaccine.Feb 17, 2022
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
Can I get COVID-19 again after having the vaccine?
Getting COVID-19 after you've been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you.Nov 9, 2021
Who should not take the Pfizer-BioNTech COVID-19 vaccine?
If you have had a severe allergic reaction to any ingredient in the Pfizer-BioNTech COVID-19 vaccine (such as polyethylene glycol), you should not get this vaccine. If you had a severe allergic reaction after getting a dose of the Pfizer-BioNTech COVID-19 vaccine, you should not get another dose of an mRNA vaccine.
What is the main ingredient in an mRNA coronavirus vaccine?
mRNA – Also known as messenger ribonucleic acid, mRNA is the only active ingredient in the vaccine. The mRNA molecules contain the genetic material that provide instructions for our body on how to make a viral protein that triggers an immune response within our bodies.Jan 11, 2021
What is a monoclonal antibody?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells.Mar 31, 2022
Are antibiotics effective in preventing or treating COVID-19?
Antibiotics do not work against viruses; they only work on bacterial infections. Antibiotics do not prevent or treat COVID-19, because COVID-19 is caused by a virus, not bacteria. Some patients with COVID-19 may also develop a bacterial infection, such as pneumonia.Mar 31, 2022
How can convalescent plasma be used to treat COVID-19?
The blood from people who recover from COVID-19 contains substances called antibodies, which are capable of fighting the virus that causes the illness. For some other diseases caused by respiratory viruses, giving people the liquid portion of blood that contains these antibodies, called plasma, obtained from those who have recovered from the virus, may lead to more rapid improvement of the disease. Patients with COVID-19 may improve faster if they receive plasma from those who have recovered from COVID-19, because it may have the ability to fight the virus that causes COVID-19.Dec 28, 2021
What is monoclonal antibody?
Monoclonal antibodies are antibodies that have a high degree of specificity (mono-specificity) for an antigen or epitope. Monoclonal antibodies are typically derived from a clonal expansion of antibody producing malignant human plasma cells. The initial monoclonal antibodies were created by fusing s …. Monoclonal antibodies are antibodies that have ...
How many daltons are in a monoclonal antibody?
Monoclonal antibodies are generally well tolerated. Because they are large proteins (typically 150-200,000 daltons in size) they require parenteral, often intravenous, administration. Circulating proteins are metabolized by many cells, but particularly by hepatocytes.
How are proteins broken down?
Proteins are broken down by cellular proteases into small peptides and amino acids that can used to synth esize other proteins . Metabolism of proteins does not generate toxic intermediates and, therefore, monoclonal antibodies are unlikely to induce drug induced liver injury via production of toxic metabolites.
What are monoclonal antibodies used for?
At present, its annual market reaches 150 billion US dollars. It is widely used in the fight against cancer, inflammatory and autoimmune diseases. Therapeutic monoclonal antibodies have also been used to treat infectious diseases . Palivizumab is the first monoclonal antibody approved for infectious disease. In 1998, the FDA authorized palivizumab to prevent serious lung disease caused by the respiratory syncytial virus in infants 61. Since then, more and more therapeutic monoclonal antibodies have been developed for quite a few infectious diseases, including some emerging infectious diseases 62, 63. The development of therapeutic monoclonal antibodies is currently at the front line of fighting against the COVID-19 pandemic 36. According to their targets, we divide the COVID-19 therapeutic monoclonal antibodies into anti-virus and anti-host categories.
When did polyclonal antibodies become popular?
Therapeutic polyclonal antibodies have been applied to treat infectious diseases since the 1890s. In 1901 , Emil Behring received the first Nobel Prize in Physiology or Medicine for his work on serum therapy, especially its application against diphtheria. Later on, convalescent sera or plasma therapies have been applied to various infectious diseases. Due to the increasing number of SARS-CoV-2 infections and the lack of effective therapies, convalescent plasma therapy has become very popular currently.
What is ADE in a virus?
Antibody-dependent enhancement (ADE) is an unexpected phenomenon that happened after vaccination or antibody therapies, where the production or presence of specific antibodies may enhance rather than inhibit viral infection 110. ADE has been observed in over 40 kinds of viruses, including two well-known coronaviruses: SARS-CoV and MERS-Cov 110. A common mechanism of ADE is that viral-specific antibody promotes viral entry into host granulocytes, monocytes, macrophages, dendritic cells, and B cells through the Fc receptor (FcR) and complement receptors 110, 111. ADE in SARS-CoV-2 has not yet been validated experimentally, but it may exist. Human lymphoid tissues and many immune cells usually lack ACE2 expression 112. A recent single-cell RNA sequencing study with 284 samples from 196 COVID-19 patients and controls has created a comprehensive immune landscape with 1.46 million cells. The data however reveal that SARS-CoV-2 RNAs exist in many immune cell types, including granulocytes, macrophages, plasma cells, T cells, and Natural Killer cells, indicating ADE or new routes for the virus entry other than ACE2 receptor 113. ADE has two faces. One is bad, injuring immune cells, amplifying the infection, and triggering harmful immunopathology. Another may be good, promoting antigen presentation and protective immune response. However, the bad one has become a common challenge for the development of vaccines and antibody therapies 114, 115. This is especially true for vaccine development, where the immune response largely relies on the genetic background of individuals and is hard to predict. For antibody development, in vitro assays and in vivo models for ADE risk evaluation are expecting to be built. As monoclonal antibodies are easy to be engineered, quite a few strategies such as Fc engineering and antibody cocktails may bypass or inhibit ADE.
What is a therapeutic cocktail?
Therapeutic cocktail antibodies are combinations of two or more monoclonal antibodies. As all its components are clearly identified and characterized, an antibody cocktail holds all advantages of monoclonal antibodies. Moreover, it targets more than one epitope or even binds multiple antigens like polyclonal antibodies. The synergism and complementarity of each monoclonal antibody make a cocktail a better choice for treating varying infectious diseases 103. For example, a combination of three monoclonal antibodies called Zmapp exceeds the efficacy of any other therapeutics against the Ebola virus, including the Guinean variant of Ebola 104.
When did bamlanivimab get approved?
On November 9, 2020, the FDA issued bamlanivimab an emergency use authorization (EUA) for the treatment of recently diagnosed mild to moderate COVID-19 in patients who are older than 12 years old, weigh at least 40 kg, and are at high risk of progressing to severe disease and/or hospitalization 91.
Is convalescent plasma safe?
Some early results show convalescent plasma therapy is not only safe but also may help eliminate the virus and improve clinical symptoms 41 ,43-45. On August 23, 2020, the U.S. Food and Drug Administration (FDA) authorized the emergency use of convalescent plasma for the treatment of hospitalized patients with COVID-19.
What is a monoclonal antibody?
Monoclonal antibodies (mAbs) are antibodies developed in a laboratory to help our bodies fight infection. Nearly 100 mAbs are FDA-approved to treat health conditions including cancers and autoimmune diseases. Monoclonal antibodies are also being studied for the treatment and prevention of COVID-19. They are given through intravenous infusion (i.e., ...
Why are antibodies made?
Antibodies are naturally made in our bodies to fight infection. Without antibodies, a virus can enter and infect a cell. With antibodies, however, when the virus tries to enter the cell, antibodies block the virus. Monoclonal antibodies (mAbs) are antibodies developed in a laboratory to help our bodies fight infection.
What is the purpose of monoclonal antibodies?
Monoclonal antibodies targeting the S protein have the potential to prevent SARS-CoV-2 infection and to alleviate symptoms and limit progression to severe disease in patients with mild to moderate COVID-19, particularly in those who have not yet developed an endogenous antibody response. 3.
What antibody targets the RBD of the S protein?
Bamlanivimab (also known as LY-CoV555 and LY3819253) is a neutralizing monoclonal antibody that targets the RBD of the S protein of SARS-CoV-2. Etesevimab (also known as LY-CoV016 and LY3832479) is another neutralizing monoclonal antibody that binds to a different but overlapping epitope in the RBD of the SARS-CoV-2 S protein. Casirivimab (previously REGN10933) and imdevimab (previously REGN10987) are recombinant human monoclonal antibodies that bind to nonoverlapping epitopes of the S protein RBD of SARS-CoV-2.
What are the four major structural proteins in the SARS genome?
The SARS-CoV-2 genome encodes four major structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N), as well as nonstructural and accessory proteins. The S protein is further divided into two subunits, S1 and S2, that mediate host cell attachment and invasion. Through its receptor-binding domain (RBD), ...
What are the adverse events of bamlanivimab?
In the Phase 2 Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) trial, the most common adverse events associated with bamlanivimab were nausea, diarrhea, dizziness, headache, pruritis, and vomiting. The safety profile of bamlanivimab at all three doses was reportedly like that of the placebo.
What is Casirivimab plus imdevimab?
Casirivimab plus imdevimab: These are recombinant human monoclonal antibodies that bind to nonoverlapping epitopes of the spike protein RBD of SARS-CoV-2. Sotrovimab: This monoclonal antibody was originally identified in 2003 from a SARS-CoV survivor.
Is bamlanivimab still available in the US?
Because of an increasing number of reports of SARS-CoV-2 variants that are resistant to bamlanivimab alone, FDA has recently revoked the EUA for bamlanivimab, and the product will no longer be distributed in the United States. 4.