
Potassium chloride is also essential to the healthy growth of both plant and animal life, including humans. Adding potassium chloride to water has been proven to remove up to 90% of the sodium present. Reverse Osmosis
Reverse osmosis
Reverse osmosis (RO) is a water purification technology that uses a semipermeable membrane to remove ions, molecules, and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, a colligative property, that is driven by c…
How do you remove sodium from well water?
Distillation – Distillation can also be used to remove sodium from drinking water. Distillation pitchers are an attractive way to remove sodium from well water, but they are costly, and output is limited. Distillation pitchers are suitable for individual use as they need a lot of time to distill the water.
How to remove salt from water naturally?
The most common and effective way to remove salt from water is through physical filtration. Specifically, reverse osmosis systems are capable of removing salt and a wide variety of other contaminants from softened water. Let’s take a closer look at reverse osmosis systems, which offer a method for how to remove salt from water naturally.
How does reverse osmosis remove sodium from water?
Reverse osmosis is a highly effective and natural method for removing sodium from softened water. At the same time, reverse osmosis systems also dramatically reduce the number of contaminants in water. Reverse osmosis systems may also contain a pre-filter to clear out any sediment and a post-filter consisting of activated carbon.
Do I need to remove sodium and chloride from my water?
While it is effective, there are some hazardous chemicals required in running the system. Most people do not need to remove sodium and chloride from their home’s water supply for health reasons, but if you have concerns about these substances in your water, contact Skillings & Sons for a consultation.
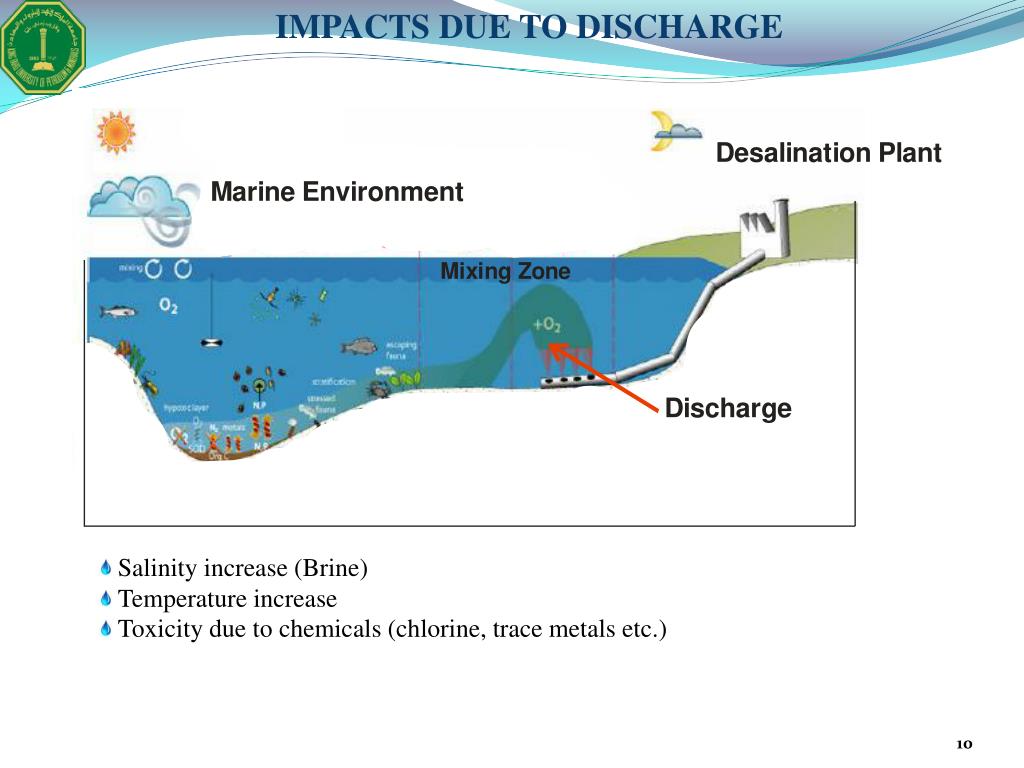
How do you remove salt from treated water?
The most common and effective way to remove salt from water is through physical filtration. Specifically, reverse osmosis systems are capable of removing salt and a wide variety of other contaminants from softened water.
What filter removes sodium from water?
Reverse Osmosis (RO)Reverse Osmosis (RO) The best part is that it can remove between 94% and 98% sodium content in your water. It is important to note that RO is one of the best options to eliminate contaminants from the water. In fact, this filtration system can also remove harmful waterborne bacteria.
Can you completely remove salt from water?
Humans cannot drink saline water, but, saline water can be made into freshwater, for which there are many uses. The process is called "desalination", and it is being used more and more around the world to provide people with needed freshwater.
Does a carbon filter remove sodium?
Activated carbon filters, which are used in the carafe-style and faucet-mounted filters that make up a significant portion of the retail filter market, do not filter sodium and other impurities, such as arsenic.
Does RO remove sodium?
Reverse Osmosis Systems will remove common chemical contaminants (metal ions, aqueous salts), including sodium, chloride, copper, chromium, and lead; may reduce arsenic, fluoride, radium, sulfate, calcium, magnesium, potassium, nitrate, and phosphorous.
Which method is commonly used to separate salt from water?
Simple distillation is a method for separating the solvent from a solution. For example, water can be separated from salt solution by simple distillation. This method works because water has a much lower boiling point than salt.
How do you change saltwater to freshwater?
Thermal distillation involves heat: Boiling water turns it into vapor—leaving the salt behind—that is collected and condensed back into water by cooling it down. The most common type of membrane separation is called reverse osmosis. Seawater is forced through a semipermeable membrane that separates salt from water.
What equipment do you need to separate salt from water?
Separate Salt and Water Using Distillation If you want to collect the water, you can use distillation. This works because salt has a much higher boiling point than water. One way to separate salt and water at home is to boil the salt water in a pot with a lid.
How Does Salt Get Into Drinking Water?
Sodium chloride is a naturally occurring element in seawater, surface water, and groundwater.
How Do You Remove Sodium From Softened Water?
We have established that soft water has a higher sodium content than hard water.
Conclusion
Water is becoming an increasingly limited commodity and we need to find economically viable ways of conserving our water resources.
How to remove salt from water?
The most common and effective way to remove salt from water is through physical filtration. Specifically, reverse osmosis systems are capable of removing salt and a wide variety ...
How does salt affect water softener?
There are two primary ways that the impact of hard water is dealt with in a residential setting: water softeners and water conditioners. A water softener removes the mineral content of hard water and replaces it with a small number of sodium ions, while a water conditioner changes the minerals so they can’t cause scaling. Water conditioners are sometimes known as salt-free water softeners since the water conditioned by these systems doesn’t contain sodium. Learn the difference between a salt versus salt free water softener with our guide.
What happens when a mineral ion is removed from a water molecule?
When these mineral ions are drawn away from the water molecule they are replaced with the sodium ions which had previously been attached to the resin beads. The replacement of a positively charged mineral ion with a positively charged sodium ion allows the water molecule to retain an ionic balance.
What happens if you don't flush your resin tank?
If these ions are not flushed from the system then eventually it will stop softening water. In order to flush the system, salty brine in the brine tank is flooded into the resin tank. The salty water displaces the mineral ions from the resin, ...
Why use both soft water and hard water?
Using both systems in this way allows you to have the benefits of both soft water and water filtration. Softening your water ensures you won’t have to deal with the soap scum, scaling, and deposits that come with hard water.
Why are some people skeptical about water softeners?
Some people are skeptical about water softeners which use ion-exchange because of the sodium in the soft water they produce. This is understandable, particularly because the dangers of high-sodium diets have been discussed extensively over recent decades.
How much sodium is in a gallon of water?
Once softened this water will contain only 74 milligrams (mg) of sodium in a quart of water or 298 mg per gallon. To put this number in perspective, each gallon of soft water provides slightly more sodium than two slices of white bread or two cups of milk.
How is sodium bicarbonate introduced into water?
Sodium bicarbonate is introduced into a filter that water circulates through. The reaction strips dissolved calcium hydroxide out of the water, where the calcium hydroxyl group bonds to the two carbon atoms in sodium bicarbonate, making calcium carbonate and a free sodium ion. The calcium carbonate is then precipitated out ...
Why is sodium bicarbonate used in triple treatment?
Because the sodium bicarbonate water treatment method results in a non-soluble precipitate, the water stream typically needs filtration. In triple treatment systems, the sodium bicarbonate treatment is usually the middle treatment in the process, after charcoal and ionic filtering (typically combined into one step) and before placer filtration ...
What is sodium bicarbonate?
Sodium bicarbonate water treatment is one part of municipal water processing. Sodium bicarbonate is a water treatment method used to soften water (removing calcium and magnesium impurities from it) in older water softener systems.
What is the end result of sodium bicarbonate?
The chief complaints are that the end result of the sodium bicarbonate process is a lot of dissolved sodium, which leaches chlorine atoms from PVC pipes and creates a salt-water waste flow that increases municipal water costs. Advertisement.
Why is water softening less effective?
Because of the nature of the chemical reaction, this method of water softening is less effective when the calcium or magnesium is bonded to sulfur compounds rather than hydroxyl groups. Advertisement.
Is calcium carbonate water soluble?
The process uses a chemical reaction to convert the calcium hydroxide (or magnesium hydroxide) into calcium carbonate, which isn't water soluble. Softened water usually has elevated levels of dissolved sodium in it compared to standard tap water, which is a minor health concern.
What happens when sodium hypochlorite dissolves in water?
When sodium hypochlorite dissolves in water, two substances are formed that play a role in oxidation and the disinfection processes. These are hypochlorous acid and the less active hypochlorite ion. The pH of the water determines how much hypochlorous acid is formed.
How is sodium hypochlorite made?
Sodium hypochlorite can be produced in two ways. One is by dissolving salt in softened water, resulting in a concentrated brine. This brine is then electrolyzed to form a sodium hypochlorite solution containing 150 grams of active chlorine per liter. During this reaction hydrogen gas is also formed. The chemical also can be produced by adding chlorine gas to caustic soda, producing sodium hypochlorite, water and salt.
What is the most common disinfectant for drinking water?
Chlorine is the most common disinfection method for drinking water in North America. It comes as chlorine gas, sodium hypochlorite or calcium hypochlorite. A small community approached Okefenokee Technical College for information on sodium hypochlorite disinfection. The community had been using chlorine gas and ammonia as primary ...
What is the pH of sodium hypochlorite?
Sodium hypochlorite is a clear, slightly yellowish solution with a characteristic odor. As a bleaching agent it is usually a 5 percent sodium hypochlorite with a pH of about 11. More concentrated solutions (10 to 15 percent) have a pH of about 13. Sodium hypochlorite is unstable.
How much does chlorine evaporate?
Chlorine evaporates at a rate of 0.75 gram per day of active chlorine from solution. Sodium hypochlorite disintegrates when heated or if it contacts acids, sunlight, certain metals, and poisonous and corrosive gases, including chlorine gas.
What are the advantages of salt electrolysis?
The advantage of salt electrolysis system in formation of sodium hypochlorite is that no transport or storage of chemical is required. Another advantage of the on-site process is that chlorine lowers the pH, and no other acid is required to lower pH. The hydrogen gas produced is explosive, and as a result ventilation is required.
Why is chlorine used in drinking water?
The use of chlorine-based disinfectants in domestic drinking water has led to controversy because the process leads to formation of small quantities of harmful byproducts. In addition, transport and handling safety concerns around the use of chlorine gas have directed public opinion toward sodium hypochlorite.
Is sodium hydroxide a caustic soda?
Caustic Soda is commonly referred to Sodium Hydroxide or NaOH. Surprisingly, it can be commonly found in the home but in the industrial sense, it is mainly used for alkaline neutralization. Caustic Soda is found in all kinds of concentrations and is a common, popular way to neutralize and tame all kinds of acids. It is also considered easy to introduce to the system due to its solubility. However, at high concentrations, it is extremely hazardous to handle and several precautious must be in place to safely use in the treatment process. These would include enhanced PPE (personal protective equipment) and immediately accessible wash stations at a minimum.
Is magnesium hydroxide good for microbial wastewater?
So, comparing magnesium hydroxide, caustic soda, and lime slurry, while all can supply the required benefits, the whole treatment process should be reviewed and determine the best overall solution based on some of the side effects for each. Magnesium hydroxide can be difficult to store when not done properly but can supply significantly more alkalinity in a bio-available form to a microbial wastewater system without advers ely affecting pH . This creates a more suitable environment for bioremediation of BOD and nutrients like nitrogen and phosphorus. Moreover, because magnesium hydroxide supplies a lightweight, divalent cation, unlike the monovalent sodium in caustic and heavier calcium in lime, magnesium hydroxide helps to generate a denser, more easily dewatered sludge, with a higher percentage of cake solids – reducing waste disposal costs.
Does magnesium hydroxide dewater cake?
Moreover, because magnesium hydroxide supplies a lightweight, divalent cation, unlike the monovalent sodium in caustic and heavier calcium in lime, magnesium hydroxide helps to generate a denser, more easily dewatered sludge, with a higher percentage of cake solids – reducing waste disposal costs.
What are the technologies used in water treatment?
Those technologies include activated carbon adsorption, ion exchange resins, and high-pressure membranes. These technologies can be used in drinking water treatment facilities, in water systems in hospitals or individual buildings, or even in homes at the point-of-entry, where water enters the home, or the point-of-use, ...
How effective is nanofiltration?
This also allows nanofiltration to remove particles while retaining minerals that reverse osmosis would likely remove. Research shows that these types of membranes are typically more than 90 percent effective at removing a wide range of PFAS, including shorter chain PFAS.
What is the difference between nanofiltration and reverse osmosis?
This technology depends on membrane permeability. A standard difference between the two is that a nanofiltration membrane will reject hardness to a high degree, but pass sodium chloride; whereas reverse osmosis membrane will reject all salts to a high degree. This also allows nanofiltration to remove particles while retaining minerals that reverse osmosis would likely remove.
Can PFAS dissolve in water?
Unfortunately, the characteristics that make them useful are the reason they persist in the environment and can bioaccumulate, or build up, in our bodies and the bodies of animals. PFAS also dissolve in water, and combined with their chemical properties mean traditional drinking water treatment technologies are not able to remove them. ...
How to remove sulfate from water?
The most common method for removing high concentrations of sulfate from water is through addition of hydrated lime (Ca (OH)2), which precipitates calcium sulfate: Na 2 SO 4 + Ca (OH) 2 => CaSO 4 + 2 NaOH Calcium sulfate , which hydrates to become the common mineral gypsum, has a solubility of approximately 2000 mg/L as sulfate.
What metals can be removed from water with etchingite?
Ettringite formation can also provide a polishing effect, allowing precipitation of difficult-to-remove metals such as chromium, arsenic, selenium and cadmium, often below their respective analytical detection limits. Boron, fluoride and up to 30 percent of the chloride and nitrate in water have also been removed.
How does CESR reduce sulfate?
The CESR process can reduce the sul fate concentration in most wastewaters to less than 100 mg/L through use of a proprietary powdered reagent. Addition of the CESR reagent to lime-treated water precipitates sulfate as a nearly insoluble calcium-alumina-sulfate compound known as ettringite.
How long does it take to remove sulfate from a sulfate solution?
This sulfate removal takes 30 to 300 minutes, depending on the amount of reagent added, level of removal required and other ions in the water . Ettringite sludge is easily dewatered, and may be reused in the process as seed sludge to reduce the quantity of coagulant required.
What is the first step in sulfate precipitation?
Step 1 -- Initial Sulfate Precipitation. For wastewater with a high metals content and a sulfate concentration greater than 8000 mg/L, hydrated lime is used initially to precipitate most of the sulfate as gypsum. This precipitation occurs at a pH below which the metals will precipitate.
How long does it take for sulfate to precipitate?
A mixing time of 40 to 60 minutes is adequate for initial sulfate precipitation.
How much CO2 is needed to reduce pH?
Recarbonation (addition of carbon dioxide) is the process typically used for pH reduction. Approximately 2 pounds of CO2 are required per 1000 gallons of water for reduction to pH 8.5. This reduction yields approximately 4 pounds of calcium carbonate and aluminum hydroxide sludge per 1000 gallons of treated water.
