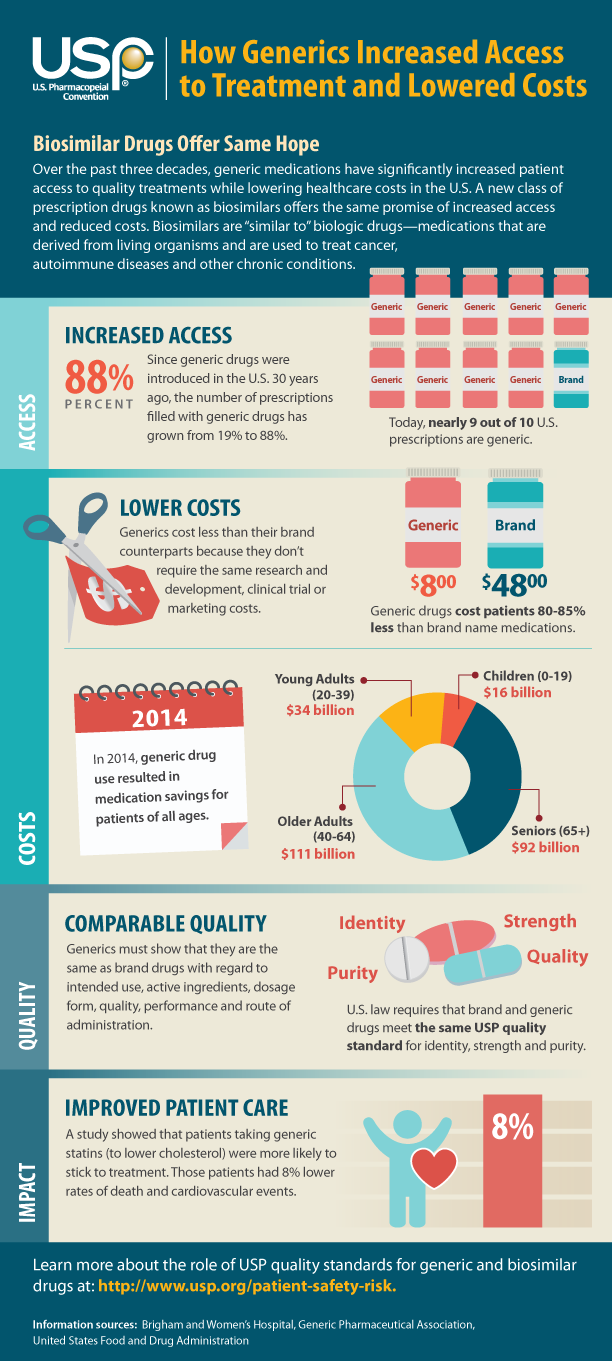
If you qualify for mAb treatment, there are three steps to get it:
- Test positive for COVID-19 sometime in the last 10 days.
- Get a referral for mAb treatment from your healthcare professional. If you do not have a healthcare professional, call the Combat COVID Monoclonal Antibodies Call Center at 1-877-332-6585 to find ...
- Locate an infusion center that’s available near you.
Why do biologics end with Mab?
Consistent with existing payment methodologies for the care setting where you provide the treatment; For COVID-19 monoclonal antibody products administered before May 6, 2021, the Medicare payment rate is approximately $310. Medicare will establish codes and rates for administering new products as the FDA approves or authorizes each product.
Where to get Mab infusion?
Jan 06, 2022 · “Some people will still be hospitalized for COVID-19. The most effective thing you can do is get vaccinated and to wear a mask.” If you think you may qualify for monoclonal antibody therapy and want to ask about getting treatment, contact your health care provider. If you live in Utah, you may fill out this questionnaire to see if you qualify.
What are Mab drugs?
Nov 03, 2021 · You must qualify for or have a doctor’s referral to get mAb treatment. Do not show up to a treatment location without an appointment. Call the state hotline at 1-800-456-7707 (6am – 11pm, 7 days a week) to find an infusion site near you. Learn more at Coronavirus.Utah.Gov
Can monoclonal antibodies kill you?
Get Treated. Crush Covid. Monoclonal antibody therapy is administered by IV infusion. The antibodies in the treatment mimic your body’s natural response to COVID-19, helping to boost your immune system. This can help stop the virus from entering your cells, limiting the spread of infection and preventing your symptoms from progressing.
COVID-19 VEKLURYTM (remdesivir)
Following the recent statement from the National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel about therapies for the COVID-19 Omicron variant, CMS created HCPCS code J0248 for VEKLURY™ (remdesivir) antiviral medication when administered in an outpatient setting.
COVID-19 Monoclonal Antibody Products
The FDA authorized the following investigational monoclonal antibody product under EUA for pre-exposure prophylaxis of COVID-19:
Important Update about Viral Variants
On April 16, 2021, the FDA revoked the EUA for bamlanivimab, when administered alone , due to a sustained increase in COVID-19 viral variants in the U.S. that are resistant to the solo product.
Medicare Coverage for COVID-19 Monoclonal Antibody Products
During the COVID-19 public health emergency (PHE), Medicare will cover and pay for these infusions (when furnished consistent with their respective EUAs) the same way it covers and pays for COVID-19 vaccines.
Coding for the Administration of COVID-19 Monoclonal Antibody Products
CMS identified specific code (s) for each COVID-19 monoclonal antibody product and specific administration code (s) for Medicare payment:
Medicare Payment for Administering COVID-19 Monoclonal Antibody Products
To ensure immediate access during the COVID-19 PHE, Medicare covers and pays for these infusions and injections in accordance with Section 3713 of the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) .
Billing for Administering COVID-19 Monoclonal Antibody Products
Health care providers can bill on a single claim for administering COVID-19 monoclonal antibody products, or submit claims on a roster bill.
What are monoclonal antibodies?
Our bodies naturally make antibodies to fight infections. However, if you haven’t received the COVID-19 vaccine or had a previous COVID-19 infection, your body will not have antibodies designed to recognize a new virus like SARS-CoV-2.
How does monoclonal antibody therapy help?
Monoclonal antibody therapy is a way of treating COVID-19 for people who have tested positive, have had mild symptoms for seven days or less, and are at high risk for developing more serious symptoms.
Who is eligible for monoclonal antibody therapy?
Given that COVID-19 vaccination provides strong protection against severe disease and need for hospitalization, monoclonal antibody therapy is an option for certain high-risk patients with COVID-19.
What is MAB treatment?
MAB treatment is for people who may have a greater risk of hospitalization due to COVID-19. Per FDA and state guidelines, only a select group of patients are eligible to receive the infusion.
What are the requirements for MAB?
MAB treatment is for people who may have a greater risk of hospitalization due to COVID-19. Per FDA and state guidelines, only a select group of patients are eligible to receive the infusion.#N#Qualifying patients must have experienced mild or moderate symptoms of COVID-19 within the past seven days and also meet one or more of the following criteria: 1 Age 65 and older 2 Body mass index (BMI) of 35 and higher 3 Chronic kidney disease 4 Diabetes 5 Compromised immune system due to cancer diagnosis or organ transplant 6 Chronic respiratory disease (for patients age 55 and older) 7 Cardiovascular disease (for patients age 55 and older)
How does monoclonal antibody therapy work?
Monoclonal antibody therapy is administered by IV infusion. The antibodies in the treatment mimic your body’s natural response to COVID-19, helping to boost your immune system. This can help stop the virus from entering your cells, limiting the spread of infection and preventing your symptoms from progressing.
Does Wellstar offer MAB?
While MAB therapy is only being offered at select locations, all other Wellstar Urgent Care Centers can evaluate a patient's condition and refer them to one of the five health parks for treatment.
Protect Yourself
mAbs can protect people from infection, worsening disease, and potential hospitalization.
Authorized by the FDA
For emergency use for those at risk of getting more serious symptoms or with high-risk COVID factors.
Protect Yourself
mAbs can protect people from infection, worsening disease, and potential hospitalization.
Authorized by the FDA
For emergency use for those at risk of getting more serious symptoms or with high-risk COVID factors.
mAbs Treatment
You’ll receive a home treatment visit. Receiving mAbs intravenously takes between 20-50 min, and injections take about 3 minutes. There is a 1 hour observation period following the treatment.
How to contact MDH for mAbs?
For questions on mAbs and variants, please contact the MDH Provider Hotline at 651-201-5414, option 3. Please visit the MDH Health Advisory: SARS-CoV-2 Variant Surveillance for questions on submitting specimens for genomic sequencing.
Can you use Regen-CoV with etesevimab?
In addition, FDA recommends that health care providers nationwide use alternative authorized monoclonal antibody therapies (REGEN-COV, sotrovimab) and not use bamlanivimab/etesevimab. This is because of the increasing prevalence of the SARS-CoV-2 Gamma (P.1) and Beta (B.1.351) variants. These two variants are not thought to be susceptible ...
