Resource Use and Unit Costs Data
| CF registry data item | Name | Units per year | Unit price ($) | Unit cost per annum ($) |
| Oral/inhaled antibiotics (as required) | Amoxycillin with clavulanic acid a | 8 | 13 | 105 |
| Oral/inhaled antibiotics (continuous) | Amoxycillin with clavulanic acid a | 52 | 13 | 668 |
| Pulmozyme | Dornase alfa (Pulmozyme) a | 12 | 814 | 9770 |
| Pancreatic enzyme (age≤10 y) | Creon 5000 a | 11 | 84 | 918 |
How much does it cost for cystic fibrosis medicine cost?
Vertex Pharmaceuticals Inc has priced its three-drug combination for cystic fibrosis (CF) at $311,503 per year, after winning early U.S. approval on Monday.
What is being done to cure cystic fibrosis?
in England It means more than 1,300 children with cystic fibrosis, aged six to 11, are newly eligible for this treatment. They will be able to start receiving it within weeks. Rare Roman wooden figure uncovered by HS2 archaeologists in Buckinghamshire ...
What is the lifespan of cystic fibrosis?
Cystic fibrosis is a serious, life-threatening disease that significantly shortens a person’s lifespan. Fortunately, with advances in treatment, many people with CF are now living into their 40s and 50s, and babies born with CF today can expect to live into their 50s and 60s.
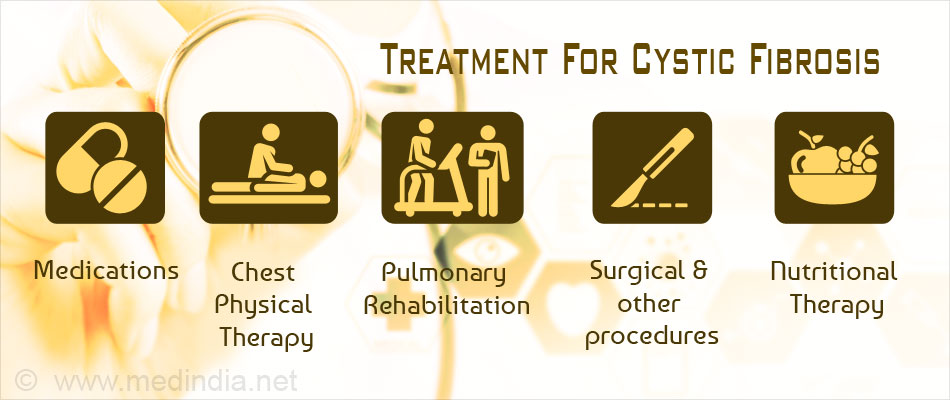
What are the primary treatment goals of cystic fibrosis (CF)?
- Increasing the frequency of airway clearance
- Inhaled bronchodilator treatment (especially if bronchial hyperresponsiveness is present or as part of airway clearance [inhaled bronchodilator followed by chest physical therapy and postural drainage])
- Chest physical therapy and postural drainage

How much is the new cystic fibrosis drug?
Trikafta costs approximately $300,000 per patient per year. Health Canada approved Trikafta in June.
How much does it cost to treat cystic fibrosis in Australia?
A French study of 65 patients treated in two medical centres estimated that the annual treatment costs were around AU$32,504 (Horvais, Touzet et al.
How much does Trikafta cost per year?
Trikafta – tezacaftor/ivacaftor/elexacaftor After accounting for production costs, the final estimated annual cost of treatment was $5676 (Figure 1c).
What is the current treatment for cystic fibrosis?
Treatments for cystic fibrosis antibiotics to prevent and treat chest infections. medicines to make the mucus in the lungs thinner and easier to cough up. medicines to widen the airways and reduce inflammation. special techniques and devices to help clear mucus from the lungs.
How much money is spent on cystic fibrosis research?
The CF Foundation spent a total of $218.1 million on research and development as well as the CF Foundation Therapeutics Lab in 2020.
How much does it cost to test for cystic fibrosis?
The CF carrier test costs about $200 – $300 per person. You need to check with your own insurance company to see if they will pay.
Does insurance cover cystic fibrosis treatment?
Cystic fibrosis and insurance A large proportion of people with CF are covered by federal or state programs. Many also receive help from patient assistance programs, due to the condition's high cost of treatment.
How much does Trikafta cost monthly?
Trikafta Coupon & Prices - Cost $49 per month.
Is Trikafta expensive?
The cost for Trikafta oral tablet (50 mg-37.5 mg-25 mg and ivacaftor 75 mg) is around $26,405 for a supply of 84, depending on the pharmacy you visit. Prices are for cash paying customers only and are not valid with insurance plans.
What famous person has cystic fibrosis?
List of people diagnosed with cystic fibrosisNameLifeBob Flanagan(1952–1996)Travis Flores(1991—)Nolan Gottlieb(1982—)Queva Griffin(1983—2003)25 more rows
Will CF ever be cured?
Even with the promising research currently underway, new treatments or cures for CF are still likely years away. New treatments require years of research and trials before governing agencies will allow hospitals and doctors to offer them to patients.
How much does Kaftrio cost the NHS?
A new drug Kaftrio, now available on the NHS, has made a huge difference to patients like Luke Copsey who has cystic fibrosis. Its list price is a mind-blowing £100,000 per year, although the health service has negotiated an undisclosed discount with the manufacturer.
What is ACFDR data?
Our study is based on the Australian Cystic Fibrosis Data Registry (ACFDR). The longitudinal nature of this data allows us to estimate the rate of CF progression, and its large sample size allows us to calculate the rate of disease progression for various age groups and health states as well as the health care costs associated with treating CF. The results from the transition and cost analysis are then combined to arrive at the estimated lifetime costs of CF.
What is the most common genetic disease?
Cystic fibrosis (CF) is the most common life-shortening genetic disease, with an incidence of 1 in 2500 and carrier frequency of 1 in 25, among Caucasians [1]. With recent advances in treatment, most children with CF now can expect to survive into adulthood and life expectancy has improved considerably.
What is Table 6?
Table 6 provides a summary of the proportion of patients who are consuming medicines identified in the CF registry data as well as tests and medical services. The use of some medicines such as antibiotics appears to be stable across health states, but the use of most treatments rises as the disease progresses. Tests such as cystic fibrosis transmembrane conductance regulator analysis and sweat chloride tests are undertaken more often when patients are in the early severity stages of CF. This is an indication that such tests are used for the initial CF diagnosis.
What are the limitations of ACFDR?
There are several limitations in this study. First, the scope of ACFDR is not complete. Patients with CF who do not attend one of the participating treatment centers will not be captured by the data. Although a recent report by Cystic Fibrosis Australia estimates that its registry now captures around 90% of all patients [30], the percentage was lower in the data available for this study. Second, coverage of the CF registry in terms of resource use is also not complete. As a result, some health care costs such as general practitioner attendances are not included in the analysis. Similarly, CF registry data may not be comprehensive in capturing resource use associated with CF-related complications such as diabetes and liver disease. We have tried to overcome such gaps by using cost evidence from other studies, but these tend to be estimated on a population basis. It may well be the case that this is an underestimate of the true costs. This is because the cost of treating complications among patients with CF may be higher than the cost of treating similar complications in those who do not have CF and are otherwise healthy. These issues could be addressed by linking ACFDR with administrative data sets that provide detailed information on health care use and costs. The ACFDR would be further enhanced by seeking quality-of-life information from patients and carers. The addition of quality- of-life data and improved resource use data would make the ACFDR a powerful tool for future analysis, and a truly unique asset in Australia. This data set can be used to undertake important economic and outcomes research. Third, the analysis uses only 3 years of registry data, limiting our ability to examine disease progression over a longer period of time. Cystic Fibrosis Australia is currently undertaking work to enable more linkage across waves. When this work is complete, it will allow researchers to utilize the panel structure of the data more fully.
Why are there so many missing FEV 1 % values in the ACFDR?
Finally, there was a large number of missing FEV 1 % values in the ACFDR. These are most likely due to issues of data collection, rather than patient selection. Despite these high numbers, our sample captures around 80% of the CF population. As children cannot generally perform the required lung function test, FEV 1 % values are also missing for those younger than 8 years. As a result, we made an assumption about these children’s health states. An important area for future research will be to model disease progression among the very young by using alternative markers of severity [31].
How many years of data are used in the ACFDR?
Three years of data from the ACFDR were used (2003, 2004, and 2005), with de-identified data from participants that can be linked across these years. The ACFDR includes information on clinical measures, mortality, demographics, complications, and health care resource use. For more information on the ACFDR, including descriptive analysis and data items, see the reports published by Cystic Fibrosis Australia [15], [16].
What is the ACFDR?
The ACFDR contains data on the forced expiratory volume in 1 second as a percentage of predicted volume (FEV 1 %)—a standard measure of lung function. Severity of lung disease is the key to the quality and length of life [17] of patients with CF. The best recorded FEV 1 % measure in each year was used to classify health states. We chose the FEV 1 % cutoff scores for severity states 1, 2, and 3 on the basis that these were consistent with a previous US study that examined the cost of CF care [10]. We generated a separate category for patients who had received a lung transplant because these patients require medications and health care services that are in addition to standard CF care [18]. For this reason, transplant patients were assumed to remain in health state 4 unless a death was recorded. Death is the fifth and absorbing health state. Patients can therefore progress through five health states, defined as follows:
How much does it cost to do a mutation analysis?
The cost of a single mutation analysis for cystic fibrosis is about $20 and the cost of a multiple mutation analysis is around $50. Cystic fibrosis can result from over 1,000 mutations of one gene. Most cases of cystic fibrosis, however, are caused by a limited number of mutation variations. Unfortunately, the cost of additional tests ...
What is the most common test for cystic fibrosis?
Most countries now implement universal newborn screening tests for cystic fibrosis. This is the most standard and common test for cystic fibrosis. Typically, a nurse takes a very small sample of your infant's blood shortly after birth, while your infant is still in the hospital. This blood sample is then sent to a lab for a preliminary cystic ...
Does insurance cover cystic fibrosis?
Moreover, most insurance companies in the United States cover the cost of this test, as well. This means, in most instances, a newborn screening test for cystic fibrosis is free for the parent. The cost of a newborn screening test for cystic fibrosis is comparable to other newborn genetic screening tests. The standard cystic fibrosis genetic ...
Is cystic fibrosis a low cost test?
A newborn screening test for cystic fibrosis is very efficient and simple, which leads to relatively low costs per infant. Typically, the cost of this newborn screening is included in health care services in countries that have universal health care, such as in Canada.
How does an inflatable vest work?
The device includes an inflatable vest connected by flexible tubes to an air-pulse generator which rapidly inflates and deflates the vest, gently compressing and releasing the chest wall. The high-frequency chest wall oscillation or HFCWO loosens, thins out, and moves the secretion towards the central airways so that it can be cleared.
How much does a cystic fibrosis vest cost?
On average, a cystic fibrosis vest is going to cost anywhere from as little as $8,000 to as much as $20,000 without any sort of health insurance, greatly depending on the brand. For those who do have a health insurance policy, be sure to check with your provider to see what they will cover. In order for most health insurance companies ...
What is cystic fibrosis vest?
Cystic fibrosis vest overview. The cystic fibrosis vest is a medical device designed for clearing excess mucus from the patient’s lungs and is used widely in homes as well as hospitals. The vest will look similar to a water life jacket, typically made of a soft and flexible material that is similar to a blood pressure cuff.
How old do you have to be to wear a vest?
As long as a child’s chest measurement is large enough for the vest, there is no minimum age requirement.
What should be included in a vest?
A vest, depending on the manufacturer, should include the tubes, remote control, power cord and generator for the air pulse.
Does insurance cover cystic fibrosis?
In order for most health insurance companies to cover a cystic fibrosis vest, a lot of documentation will be required and will be medically necessary. The costs, if covered by your health insurance policy, will depend on your co-pays and the deductible that needs to be met. We researched a few brands of cystic fibrosis vests online ...
Do you need electricity to operate a vest?
The vests will also require electricity while you take it on the go. Since most equipment weighs close to 30 pounds, some find it tough to travel with. On the bright side, however, it won’t require much skill to operate, helping you receive treatment without professional help.
What is triple combination therapy?
The triple combination therapy is a CFTR modulator that helps the defective CFTR protein to work more effectively. While elexacaftor and tezacaftor both work as correctors — increasing the amount of functional CFTR that reaches the cell surface by helping the F508del mutated protein fold correctly — ivacaftor (also marketed independently as Kalydeco) is a potentiator that improves the flow of chloride molecules through the CFTR channel.
What is the most recent approved CF therapy?
ICER compared the efficacy and value of Trikafta ( elexacaftor , tezacaftor, and ivacaftor ), the most recent approved CF therapy marketed by Vertex Pharmaceuticals, and found that it “provides a substantial (moderate-large) net health benefit” compared to other approaches.
What is the highest evidence rating for CF?
The Institute for Clinical and Economic Review (ICER) granted the therapy Trikafta the highest evidence rating (an “A”) for the treatment of cystic fibrosis (CF) in patients with either two copies the F508del mutation, or with one copy of the F508del mutation and a minimal function mutation in the CFTR gene (the gene defective in CF).
Why was the ICER meeting postponed?
ICER had scheduled a public meeting to discuss the data, but due to COVID-19 concerns , the meeting has been postponed indefinitely.
How much does Trikafta cost?
For Trikafta, for example, an appropriate health-benefit price would range from $67,900-$85,500 per year, which would require at least a 73% discount off the therapy’s list price.
Is Trikafta better than Symdeko?
Results from these trials, along with a meta-analysis comparing the outcomes of patients treated with multiple CFTR modulators, have led ICER to consider with “high certainty” that Trikafta has a substantial benefit over best supportive care and Symdeko treatment in people with two F508del mutations.
Is Trikafta a phase 3 drug?
Trikafta has been tested in two pivotal Phase 3 clinical trials ( NCT03525444 and NCT03525548 ), in which it significantly improved measures of lung function in CF patients with either two F508del mutations or one F508del and one minimal function mutation.
