
What is chelation therapy and how does it work?
Some of the common side effects that a person may experience during chelation therapy include:
- fever and chills
- headaches
- nausea and vomiting
- diarrhea
What are the basics of chelation therapy?
Chelation therapy is a chemical process in which a synthetic solution—EDTA (ethylenediaminetetraacetic acid)—is injected into the bloodstream to remove heavy metals and/or minerals from the body. Chelation means "to grab" or "to bind." When EDTA is injected into the veins, it "grabs" heavy metals and minerals such as lead, mercury, copper ...
What are the health benefits of chelation therapy?
- Alzheimer’s disease
- Heart disease – And subsequently diabetes
- Autism
- Parkinson’s disease
- & Much More
What diseases can be treated with chelation therapy?
- Ornish D, et al. Intensive lifestyle changes for reversal of coronary heart disease. ...
- Mood MB et al. Toxic mechanisms of five heavy metals. ...
- Lanphear BP, et al. Low-level lead exposure and mortality in US adults. ...
- Frustaci A, et al. ...
- Petteruti S. ...
- https://edta.net/the-early-history-of-edta-chelation
- Clarke NE, et al. ...
- Maniscalco BS, et al. ...
- Lamas GA, et al. ...
- E, et al. ...
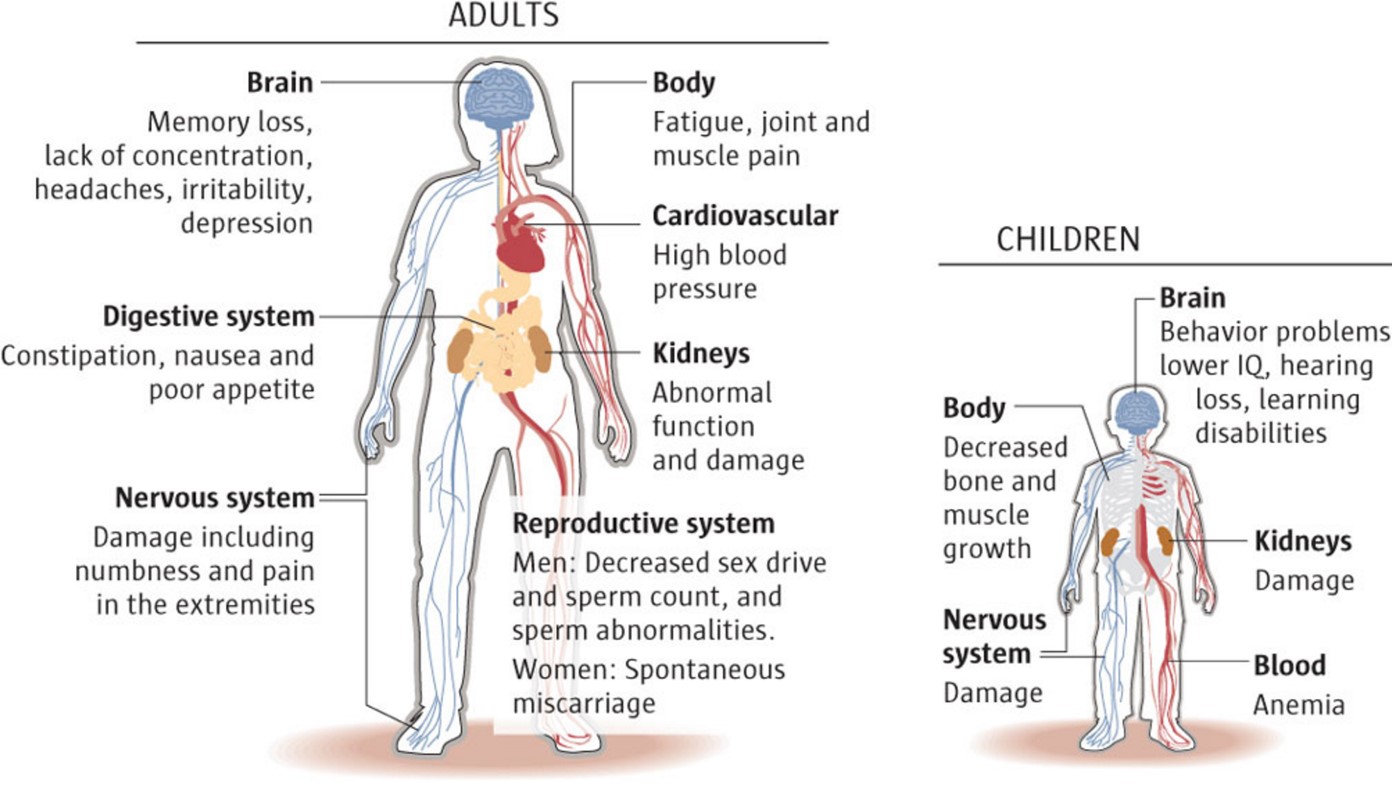
What is chelation used for?
When metals like lead, mercury, iron, and arsenic build up in your body, they can be toxic. Chelation therapy is a treatment that uses medicine to remove these metals so they don't make you sick. Some alternative health care providers also use it to treat heart disease, autism, and Alzheimer's disease.
What metals does chelation remove?
Chelation therapy is a method for removing heavy metals, such as mercury or lead, from blood. It's one of the standard treatments for many types of metal poisoning....Chelation therapy is a very effective way to remove several heavy metals from blood, including:lead.arsenic.mercury.iron.copper.nickel.
What is chelation reaction?
Chelation is the reaction between a metal ion and an organic complexing agent resulting in the formation of a ring structure that encompasses the metal ion and removes it (Olson, 2004). Dissolved organic molecules bind to metals in solution or on the surface of minerals.
What is chelation therapy for lead?
Chelation therapy. In this treatment, a medication given by mouth binds with the lead so that it's excreted in urine. Chelation therapy might be recommended for children with a blood level of 45 mcg/dL or greater and adults with high blood levels of lead or symptoms of lead poisoning.
How does a chelating agent work?
A chelating agent is a chemical compound that reacts with metal ions to form stable, water-soluble metal complexes. The agent rearranges the metal's chemical composition and improves the metal's general stability and likelihood to bond with other substances.
What is metal chelation?
Chelation therapy is the preferred medical treatment for reducing the toxic effects of metals. Chelating agents are capable of binding to toxic metal ions to form complex structures which are easily excreted from the body removing them from intracellular or extracellular spaces.
What is chelation and chelate effect?
The chelate effect is the enhanced affinity of a chelating ligand for a metal ion compared to its monodentate ligand counterpart(s). This term comes from the Greek chelos, meaning "crab". A crab does not have any teeth at all, but it does have two claws for tightly holding onto something.
What are the benefits of chelation therapy?
Chelation therapy removes metals that have built up in the body. Its proponents claim that this can rejuvenate the heart and blood vessels, improve liver and kidney function, increase blood flow to the brain, and more.
Is chelation therapy used for lead poisoning?
Some children with severe lead poisoning may need a medicine to help remove lead from their blood. Using medicine to take lead out of the blood is called chelation (key LAY shun).
Which chelate is used for lead poisoning?
Dimercaprol (British antilewisite [BAL], or 2,3-dimercapto-1-propanol) was the first chelator used in encephalopathic individuals and is the drug of choice for treatment of lead toxicity.
History
Who Does It
- Any licensed physician can perform chelation therapy on a patient. However, chelation therapy for uses other than metal toxicity is not conventionally taught in medical school, and physicians who perform it generally are naturopathic doctorsor medical doctors who receive specialized training for it.
Evidence
- The scientific support for chelation therapy's benefits for health conditions other than metal poisoning is limited. There is a consensus that much more research is needed in this area. For example, one comprehensive review of studies on chelation therapy and heart health concluded that there was insufficient evidence to determine whether chelation therapy is effective or not.1…
Side Effects & Safety Concerns
- A major reason chelation therapy is not widely accepted for conditions other than metal poisoning is due to the risk of side effects, which is significant. Side effects can especially occur when higher doses are used, and include:15 1. Diarrhea 2. Weight loss 3. High blood pressure 4. Abdominal pain 5. Gastrointestinal disorders 6. Nausea 7. Skin rash 8. Vomiting 9. Flu-like symp…
A Word from Verywell
- Chelation therapy has been effective in treating metal poisoning, but its efficacy in treating other conditions is unclear. Be sure you talk to your doctor and understand the risks of chelation therapy if you are interested in receiving it.
Overview
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's …
Medical applications
In the 1960s, scientists developed the concept of chelating a metal ion prior to feeding the element to the animal. They believed that this would create a neutral compound, protecting the mineral from being complexed with insoluble salts within the stomach, which would render the metal unavailable for absorption. Amino acids, being effective metal binders, were chosen as the prospective ligands, and research was conducted on the metal–amino acid combinations. The r…
Chelate effect
The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal.
The thermodynamic principles underpinning the chelate effect are illustrated by the contrasting affinities of copper(II) for ethylenediamine (en) vs. methylamine.
Cu + en ⇌ [Cu(en)] (1)
The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal.
The thermodynamic principles underpinning the chelate effect are illustrated by the contrasting affinities of copper(II) for ethylenediamine (en) vs. methylamine.
Cu + en ⇌ [Cu(en)] (1)
In nature
Numerous biomolecules exhibit the ability to dissolve certain metal cations. Thus, proteins, polysaccharides, and polynucleic acids are excellent polydentate ligands for many metal ions. Organic compounds such as the amino acids glutamic acid and histidine, organic diacids such as malate, and polypeptides such as phytochelatin are also typical chelators. In addition to these adventitious chelators, several biomolecules are specifically produced to bind certain metals (se…
Industrial and agricultural applications
Homogeneous catalysts are often chelated complexes. A representative example is the use of BINAP (a bidentate phosphine) in Noyori asymmetric hydrogenation and asymmetric isomerization. The latter has the practical use of manufacture of synthetic (–)-menthol.
Citric acid is used to soften water in soaps and laundry detergents. A common synthetic chelator is EDTA. Phosphonates are also well-known chelating agents. Chelators are used in water treatmen…
Etymology
The word chelation is derived from Greek χηλή, chēlē, meaning "claw"; the ligands lie around the central atom like the claws of a lobster. The term chelate was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or chele (Greek) of the lobster or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings."
See also
• Chelation therapy – Medical procedure to remove heavy metals from the body
External links
• The dictionary definition of chelate at Wiktionary