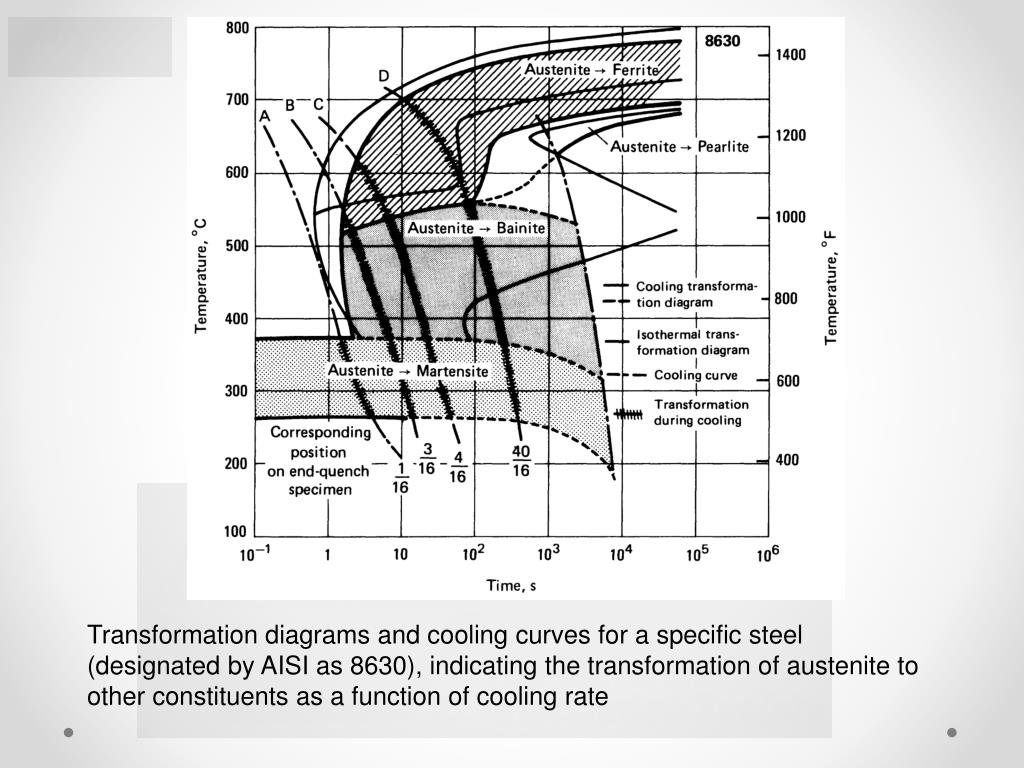
The purpose of the austenitic stainless steel solution heat treatment is to re-dissolve the alloy carbides, such as (FeCr) 23C6, and the σ phase, which are produced or precipitated in the previous processing steps, into austenite to obtain a single austenite (Some may have a small amount of δ ferrite), to ensure that the material has good mechanical properties and corrosion resistance, and fully eliminate the stress and cold hardening phenomenon.
Full Answer
What happens to austenite when it cools?
As austenite cools, the carbon diffuses out of the austenite and forms carbon-rich iron-carbide (cementite) and leaves behind carbon-poor ferrite. Depending on alloy composition, a layering of ferrite and cementite, called pearlite, may form.
What is austenite hardening process?
Austempering is a hardening process that is used on iron-based metals to promote better mechanical properties. The metal is heated into the austenite region of the iron- cementite phase diagram and then quenched in a salt bath or other heat extraction medium that is between temperatures of 300–375 °C (572–707 °F).
How does temperature affect the austenitization process?
By changing the temperature for austenitization, the austempering process can yield different and desired microstructures. A higher austenitization temperature can produce a higher carbon content in austenite, whereas a lower temperature produces a more uniform distribution of austempered structure.
Can austenitic stainless steel be strengthened by heat treatment?
Austenitic stainless steel can not be strengthened by heat treatment, but can be strengthened by cold working deformation (cold hardening, deformation strengthening), will increase the strength, plasticity decreased.
Why do you heat to the austenite temperature range?
At high enough temperatures, austenite can be stable without carbon (911 °C in Figure 1), but at these lower temperatures austenite requires dissolved carbon to be stable. On heating, therefore, austenite nucleates at the carbides, which supply carbon to the growing austenite grains.
What is austenite in heat treatment?
Austempering is a hardening process that is used on iron-based metals to promote better mechanical properties. The metal is heated into the austenite region of the iron-cementite phase diagram and then quenched in a salt bath or other heat extraction medium that is between temperatures of 300–375 °C (572–707 °F).
Why ferrite and austenite are not heat treatable?
Ferritic and austenitic stainless steels can be heat treated, but they cannot be hardened. Due to the high amount of chromium and nickel the austenitic phase of these materials is stable at room temperature – and austenite is a quite soft phase.
Why is austenite phase important?
Once austenite formation is complete, the condition of austenite as a function of heat treatment is of critical importance to the final properties of the steel. In particular, austenite grain size has an important effect on subsequent processing.
What is austenite used for?
Austenitic stainless steels are used for domestic, industrial, transport, and architectural products based primarily on their corrosion resistance but also for their formability, their strength, and their properties at extreme temperatures.
What is meant by austenitic?
Definition of austenite : a solid solution in iron of carbon and sometimes other solutes that occurs as a constituent of steel under certain conditions.
Why austenitic steel is not heat treatable?
With sufficient quantities of nickel, stainless steel remains austenite at room temperature, creating the austenitic steels. They are nonmagnetic and cannot be heat treated for through hardening like carbon steels because the phase transformation to martensite does not occur in these alloys.
Are austenitic stainless steels heat treatable?
Austenitic stainless steels cannot harden via heat treatment. Instead, these steels work harden (they attain hardness during their manufacture and formation). Annealing these stainless steels softens them, adds ductility and imparts improved corrosion resistance.
What is the difference between ferritic and austenitic steels?
The main difference between austenitic and ferritic stainless steel is that the former features a crystalline structure, whereas the latter contains a higher concentration of chromium. Austenitic stainless steel is also better protected against corrosion than ferritic stainless steel.
What happens when you quench austenite?
If you quench a carbon steel from its austenite regime, you will have phase transformation and at high cooling rates to room temperature you will have martensite and possibly retained austenite, if the carbon content is high. If the cooling is stopped at a higher temperature, retained austenite fraction is higher.
What is the difference between ferrite and austenite?
Austenite and ferrite are two allotropes of iron. The difference between austenite and ferrite is that the austenite has the face-centered cubic configuration of gamma iron whereas the ferrite has the body-centered cubic alpha iron configuration.
Is austenite a phase?
Austenite is a high temperature phase and has a Face Centred Cubic (FCC) structure [which is a close packed structure]. The alpha phase is called ferrite. Ferrite is a common constituent in steels and has a Body Centred Cubic (BCC) structure [which is less densely packed than FCC].
Why does austenite decrease strength?
The reason for the decrease in strength is probably that when the stabilized treatment is carried out, the strong carbide-forming element titanium binds to more carbon to TiC, reducing the degree of carbon in the austenite solid solution , and the TiC will also heat Agglomeration grow up, which will also have an impact on the intensity.
What is austenitic stainless steel?
Austenitic stainless steel or products (springs, bolts, etc.) by the cold deformation after strengthening, there is a large processing stress, the existence of this stress in stress corrosion environment, the use of increased stress corrosion sensitivity, The stability of the.
What is the best treatment for stainless steel?
2. Stable annealing. Stabilized annealing is a heat treatment method for austenitic stainless steels containing stabilized titanium or niobium. The purpose of this method is to use titanium, niobium and carbon strong bonding properties, stable carbon, so as not to combine with chromium, and ultimately achieve the purpose ...
Does stainless steel have a cooling rate?
The cooling and cooling rates of the austenitic stainless steels have little effect on the stabilization effect. Therefore, in order to prevent deformation of the complex parts or to minimize the stress of the workpiece, a smaller cooling rate such as air cooling or furnace cold. 3.
Can stainless steel be heated?
Austenitic stainless steel can not be strengthened by heat treatment, but can be strengthened by cold working deformation (cold hardening, deformation strengthening), will increase the strength, plasticity decreased. Austenitic stainless steel or products (springs, bolts, etc.) by the cold deformation after strengthening, ...
Does stress treatment reduce hardness?
The stress treatment not only reduces the stress of the part, but also increases the hardness and elasticity limits without significant changes in elongation. First of all, we should pay attention to the austenitic stainless steel solution treatment temperature of the reasonable choice, in the austenitic stainless sodium material standards, ...
Is sensitization a stainless steel?
The sensitization treatment does not actually belong to the austenitic stainless steel or its products in the manufacturing process should be used in the heat treatment method. But as a procedure used to test the resistance to intergranular corrosion of austenitic stainless steels. The sensitization treatment essentially makes the treatment ...
What is the temperature of austenite?
In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures.
What causes austenite in cementite?
Heating white cast iron above 727 °C (1,341 °F) causes the formation of austenite in crystals of primary cementite. This austenisation of white iron occurs in primary cementite at the interphase boundary with ferrite. When the grains of austenite form in cementite, they occur as lamellar clusters oriented along the cementite crystal layer surface. Austenite is formed by diffusion of carbon atoms from cementite into ferrite.
What is the process of annealing iron?
Austempering is a hardening process that is used on iron-based metals to promote better mechanical properties . The metal is heated into the austenite region of the iron- cementite phase diagram and then quenched in a salt bath or other heat extraction medium that is between temperatures of 300–375 °C (572–707 °F). The metal is annealed in this temperature range until the austenite turns to bainite or ausferrite (bainitic ferrite + high-carbon austenite).
Why is austenite called an allotrope?
The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902); it exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures.
What happens when you change the temperature of austempering?
A higher austenitization temperature can produce a higher carbon content in austenite, whereas a lower temperature produces a more uniform distribution of austempered structure.
Why is austenite grown on the diamond 100?
The epitaxial growth of austenite on the diamond (100) face is feasible because of the close lattice match and the symmetry of the diamond (100) face is fcc. More than a monolayer of γ-iron can be grown because the critical thickness for the strained multilayer is greater than a monolayer.
What elements are used to make stainless steel stable?
On the other hand, such elements as silicon, molybdenum, and chromium tend to de-stabilize austenite, raising the eutectoid temperature.
What happens to austenite when the temperature is raised above Ae 1?
As the temperature is raised above Ae 1, it is the pearlite which transforms to austenite first. When all the pearlite has changed to austenite, this austenite grows consuming increasing amount of free ferrite (in hypo-eutectoid steels), or free cementite (in hypereutectoid steels) as the temperature is raised above Ae 1 to above Ae 3, ...
How long does it take for austenite to form?
The rate of austenite formation increases rapidly with the increase of temperature, such as for example, at 800°C, the process of austenitisation (with some carbides in it) takes 5 seconds. On continuous heating of steel, pearlite to austenite transformation takes place over a certain temperature interval.
What temperature does pearlite turn into austenite?
Higher is the rate of heating, higher is the temperature at which pearlite starts to transform to austenite, but longer is the temperature interval of this transformation. Curve II, the transformation starts at around 750°C, but complete homogeneous austenite is obtained at 870°C within around 12 seconds.
What temperature does vanadium carbide dissolve?
For example, vanadium carbide dissolves at around 1050°C, whereas, the niobium carbide dissolves only around 1150°C, and these temperatures are much higher than required for cementite dissolution. Depending on the nature of the alloying elements, these are non-uniformly distributed between ferrite and the carbide.
What is the first step in the heat treatment cycle of steel?
The first step in the true heat treatment cycle of steel is the austenitisation, i.e. to get a homogeneous austenite by heating it to a predetermined temperature in the austenite stability range. Normally, the initial structure of carbon steels, before this heat treatment, is ferrite + pearlite (in hypo-eutectoid steels), ...
Is austenite homogeneous or homogeneous?
This austenite has undissolved carbide in it. Higher temperature and/or more time are needed to dissolve the carbide. The resultant austenite is in- homogeneous and requires higher temperatures and/or time to obtain completely homogeneous austenite.
Is austenite a carbon?
At this time, when the whole of the pearlitic structure has transformed to austenite, there is non-uniformly of carbon in austenite, i.e., it has higher carbon content at the sites formerly occupied by the cementite and lowest at the centre of former ferrite lamellae. Additional time is needed to obtain a homogeneous austenite.
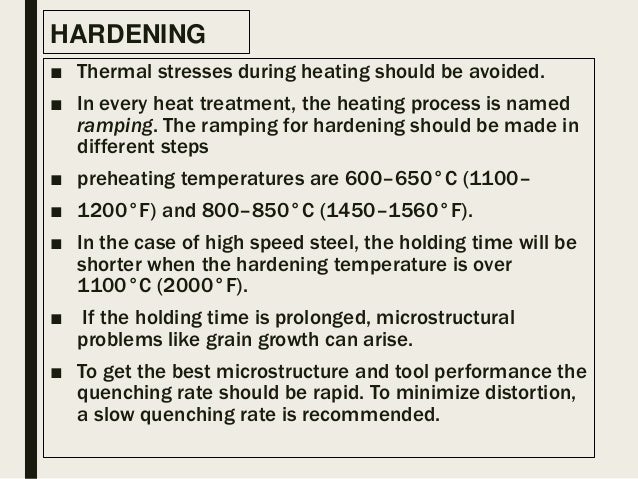
What is the effect of heat treatment?
The effect of heat treatment is small and may not be measurable. It involves the higher residual stresses created when parts are water quenched. Parts which are air cooled or gas quenched as in vacuum heat treating have less residual stress and therefore would be expected to have slightly lower hardness.
Which elements promote the formation of ferrite?
The relative amounts of these elements have a major effect on the ferrite content. Elements such as chromium, molybdenum, silicon, and columbium (niobium) promote ferrite. The higher their total content, the higher the ferrite content.
What is the temperature of stainless steel?
For these kinds of series, heating will not affect mechanical properties: but if you heat them at a temperature between (roughly) 450 - 7. Continue Reading.
What is 304 stainless steel?
Austenitic stainless steels typically have 16-26% chromium (Cr) and 8-22% nickel (Ni). Type 304, which contains approximately 18%Cr and 10%Ni, is a commonly used alloy for welded fabrications and these alloys can be readily welded using any of the arc welding processes (TIG, MIG, MMA and SA).
Is stainless steel heat treatable?
There are heat treatable stainless steels but they usually contain a martensite forming alloying element and a popular one is boron because boron doesn’t created chrome compounds that are susceptible to corrosion. Boron surface treatment also provides resistance to strong reducing agents like molten sodium.
Which elements are not equal in their power to affect the ferrite content?
Conversely, some elements (nickel, manganese, carbon, nitrogen, etc.) promote the formation of austenite: the higher their combined content, the less ferrite which is formed. These elements are not equal in their power to affect the ferrite content.
Can you heat treat stainless steel?
Other posters have listed a lot of benefits that you don’t get by heat treating most stainless steels but there is a major failure caused by heat treating a single phase steel. A typical stainless has a lot of chrome and heating below the melting point will cause chrome carbides to form and then they migrate to the grain boundary.
Why does austenite crack steel?
As the carbon content of the retained austenite is high, the martensite that forms is highly tetragonal and the resulting expansion cracks the steel because the matrix is not ductile enough to tolerate the expansion stresses.
What is the difficulty of working with a carburized specimen?
Working with a carburized specimen presents difficulties due to the variation in carbon and microstructure in the case. The writer is planning additional EBSD work with Dr. Zaefferer using 1.25-inch-diameter bars (to avoid mounting and conductivity problems) of O1 and 52100 alloy steels, high-carbon steels with enough hardenability to be through-hardened and with enough carbon and alloy content to produce >10% retained austenite. As their alloy content is not high, carbide interference peaks and texture problems by XRD should be minimal. Transverse and longitudinal specimens will be prepared and tested by XRD, then by LOM and EBSD. These tests will be performed quickly, and other labs will participate to evaluate the reproducibility of the data. Some experiments will be run at a later date to access the influence of time since heat treatment upon the data. IH
Is retained austenite bad for steel?
In the tool-steel industry, excessive retained austenite is universally considered to be detrimental. Exactly what constitutes “excessive” is difficult to define as not enough data exists, and what is excessive will vary with the grade and application. For example, relatively low-carbon 5%-Cr hot-work die steels such as H11 and H13 have been used for years as guage blocks. Any dimensional change with time is to be avoided. Consequently, these steels are triple tempered at a relatively high temperature where retained austenite will be converted to either fresh martensite or bainite, and they will be tempered with the next tempering cycle.
Can austenite be triple tempered?
Any dimensional change with time is to be avoided. Consequently, these steels are triple tempered at a relatively high temperature where retained austenite will be converted to either fresh martensite or bainite, and they will be tempered with the next tempering cycle.
Is martensite a high carbon?
Depending upon the carbon content of the parent austenite phase, either lath (low-carbon) or plate (high-carbon) martensite may form, as well as mixtures of the two. In general, lath martensite is associated with high toughness and ductility but low strength, while plate martensite structures are much higher strength but may be rather brittle and non-ductile.
Can LOM detect austenite?
LOM could not image retained austenite until it was at least present in the 10-15% range. TEM thin foils could detect and image retained austenite even at levels somewhat under 2% with careful use of dark-field illumination.
Does austenite change to martensite?
Retained austenite does become stable with time, and some will transform to martensite at room temperature. Samuels [1] states that up to 5% of the austenite present after quenching and low-temperature tempering (<200°C) will transform to martensite soon after quenching or over a period of some months.

Overview
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron, with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902); it ex…
Allotrope of iron
From 912 to 1,394 °C (1,674 to 2,541 °F) alpha iron undergoes a phase transition from body-centred cubic (BCC) to the face-centred cubic (FCC) configuration of gamma iron, also called austenite. This is similarly soft and ductile but can dissolve considerably more carbon (as much as 2.03% by mass at 1,146 °C (2,095 °F)). This gamma form of iron is present in the most commonly used type of stainless steel for making hospital and food-service equipment.
Material
Austenitization means to heat the iron, iron-based metal, or steel to a temperature at which it changes crystal structure from ferrite to austenite. The more open structure of the austenite is then able to absorb carbon from the iron-carbides in carbon steel. An incomplete initial austenitization can leave undissolved carbides in the matrix.
For some iron metals, iron-based metals, and steels, the presence of carbides may occur during …
Austempering
Austempering is a hardening process that is used on iron-based metals to promote better mechanical properties. The metal is heated into the austenite region of the iron-cementite phase diagram and then quenched in a salt bath or other heat extraction medium that is between temperatures of 300–375 °C (572–707 °F). The metal is annealed in this temperature range until the austenite turns to bainite or ausferrite (bainitic ferrite + high-carbon austenite).
Behavior in plain carbon-steel
As austenite cools, the carbon diffuses out of the austenite and forms carbon-rich iron-carbide (cementite) and leaves behind carbon-poor ferrite. Depending on alloy composition, a layering of ferrite and cementite, called pearlite, may form. If the rate of cooling is very swift, the carbon does not have time enough to diffuse and the alloy may experience a large lattice distortion known as martensitic transformation in which it transforms into martensite, a body centered tetragonal stru…
Behavior in cast iron
Heating white cast iron above 727 °C (1,341 °F) causes the formation of austenite in crystals of primary cementite. This austenisation of white iron occurs in primary cementite at the interphase boundary with ferrite. When the grains of austenite form in cementite, they occur as lamellar clusters oriented along the cementite crystal layer surface. Austenite is formed by diffusion of carbon atoms from cementite into ferrite.
Stabilization
The addition of certain alloying elements, such as manganese and nickel, can stabilize the austenitic structure, facilitating heat-treatment of low-alloy steels. In the extreme case of austenitic stainless steel, much higher alloy content makes this structure stable even at room temperature. On the other hand, such elements as silicon, molybdenum, and chromium tend to de-stabilize austenite, raising the eutectoid temperature.
Austenite transformation and Curie point
In many magnetic ferrous alloys, the Curie point, the temperature at which magnetic materials cease to behave magnetically, occurs at nearly the same temperature as the austenite transformation. This behavior is attributed to the paramagnetic nature of austenite, while both martensite and ferrite are strongly ferromagnetic.