Full Answer
Who is at risk for getting HIV?
Jul 16, 2021 · These guidelines bring in the most recent guidance on HIV testing strategies - the entry point for HIV prevention and treatment - and include comprehensive guidance on infant diagnosis. Key recommendations are presented on rapid antiretroviral therapy (ART) initiation and the use of dolutegravir. Updated recommendations are included on the timing of ART for …
What you should know about HIV treatment?
Guidelines are statements that include recommendations developed using a systematic process based on prevailing guideline development standards. The Division of HIV Prevention (DHP) within the National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP) has been at the forefront in developing guidelines on HIV prevention and care with significant national …
Who recommends dolutegravir for HIV?
Jul 22, 2019 · Based on new evidence assessing benefits and risks, the WHO recommends the use of the HIV drug dolutegravir (DTG) as the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.
Who is at greatest risk for HIV transmission?
The CDC and other organizations share recommendations for HIV prevention,treatment, and statistics that help inform new advancements from research. The latest materials provide guidance for clinicians, public health professionals, and people who may be at risk.
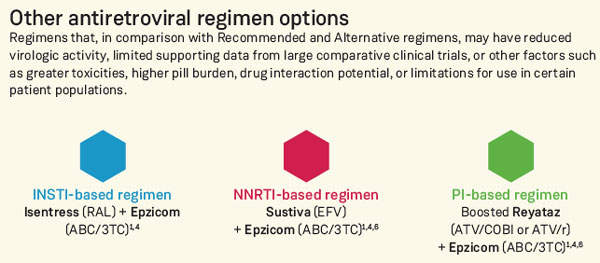
WHO recommended drugs for HIV?
Update on recommendations on antiretroviral regimens for treating and preventing HIV infection: In 2016, WHO published the consolidated guidelines on the use of antiretroviral (ARV) drugs for treating and preventing HIV infection and recommended tenofovir disoproxil fumarate (TDF) + lamivudine (3TC) (or emtricitabine, ...Jan 1, 2018
What is the recommended treatment of HIV infection?
The treatment for HIV is called antiretroviral therapy (ART). ART involves taking a combination of HIV medicines (called an HIV treatment regimen) every day. ART is recommended for everyone who has HIV.Aug 16, 2021
WHO recommended drugs for HIV prophylaxis?
PEP RegimenDrug nameDrug classEmtricitabine (Emtriva; FTC)NRTIEtravirine (Intelence; ETR)NNRTILamivudine (Epivir; 3TC)NRTILopinavir/ritonavir (Kaletra; LPV/RTV)PI9 more rows•Sep 22, 2021
What is the current recommendation by the CDC regarding HIV testing?
CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. For those at higher risk, CDC recommends getting tested at least once a year.
When is PEP not recommended?
PEP is not recommended when care is sought >72 hours after exposure. What Is PEP? National Guidelines published by the Centers for Disease Control and Prevention (CDC) in 2005 were updated in April of 2016.
Is PrEP and pep the same drug?
PrEP stands for pre-exposure prophylaxis and PEP stands for post-exposure prophylaxis. Prophylaxis means “treatment or actions taken to prevent a disease.” PrEP is a treatment plan to prevent HIV before a person is exposed while PEP is a treatment plan for after a person is exposed.May 12, 2020
Who needs PEP?
PEP may be right for you if you are HIV-negative or don't know your HIV status, and you think you may have been exposed to HIV in the last 72 hours: During sex (for example, you had a condom break with a partner of unknown HIV status or a partner with HIV who is not virally suppressed)Apr 28, 2021
What are the revised recommendations for HIV testing?
Revised Recommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health Care Settings#N#These revised recommendations provide guidance for HIV testing of adults, adolescents, and pregnant women in health care settings.
What are the guidelines and related implementation resources?
The listed guidelines and related implementation resources provide guidance about prevention strategies and services that can prevent or diagnose new HIV infections and link individuals at risk to relevant prevention, medical, and social services.
What is the preferred first line treatment for HIV?
WHO recommends dolutegravir as preferred HIV treatment option in all populations. Based on new evidence assessing benefits and risks, the WHO recommends the use of the HIV drug dolutegravir (DTG) as the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.
What are the guidelines group considered?
The guidelines group also considered mathematical models of the benefits and harms associated with the two drugs; the values and preferences of people living with HIV, as well as factors related to implementation of HIV programmes in different countries, and cost.
Why is informed choice important?
As for any medications, informed choice is important. Every treatment decision needs to be based on an informed discussion with the health provider weighing the benefits and potential risks.
What is the threshold for drug resistance?
In 2019, 12 out of 18 countries surveyed by WHO reported pre-treatment drug resistance levels exceeding the recommended threshold of 10%. All of above findings informed the decision to update the 2019 guidelines.
Is DTG a drug?
DTG is a drug that is more effective, easier to take and has fewer side effects than alternative drugs that are currently used. DTG also has a high genetic barrier to developing drug resistance, which is important given the rising trend of resistance to EFV and nevirapine-based regimens.
What is the importance of HIV prevention?
An essential part of a HIV prevention strategy is ensuring the people you serve have the latest information on diseases that may affect them. The CDC and other organizations share recommendations for HIV prevention,treatment, and statistics that help inform new advancements from research.
What is the CDC's guidance?
CDC offers guidelines and recommendations on a variety of topics related to HIV and AIDS awareness and prevention. Here is a comprehensive list of CDC’s guidance for prevention partners, clinicians, and those who may be at risk.
4.1. Introduction
In 2016, WHO strongly recommended initiating ART for all adults living with HIV regardless of WHO clinical stage and at any CD4 cell count. This “treat-all” recommendation has resulted in the scale-up of ART in more than 130 countries globally, accompanied by increased levels of availability of treatment monitoring.
4.2. Preparing people living with HIV for ART
Before people start ART, health-care providers should initiate a detailed discussion about their willingness and readiness to initiate ART, the choice of ARV drug regimen, dosage, scheduling, likely benefits, possible adverse effects and required follow-up and monitoring visits.
4.3. What to expect in the first months of ART
Although ART is a lifelong commitment, the first months are especially important.
4.4. When to start ART
ART should be initiated for all people living with HIV regardless of WHO clinical stage and at any CD4 cell count.
4.8. Monitoring ARV toxicity
The availability of laboratory monitoring is not required for initiating ART.
4.9. ARV drug resistance
A high prevalence of pretreatment HIV drug resistance to NNRTIs negatively affects the success of the public health response to HIV and potentially endangers the attainment of the global target to end the AIDS epidemic as a global threat.
4.10. Key ARV drug interactions
Drug interactions can reduce the efficacy of ART and/or increase ART-related toxicity. This section summarizes the major ARV drug interactions. A detailed table on drug interactions is available in the annexes.