
High Impact Research Articles
| Publication Title | Author Listing |
| Publication Title | Author Listing |
| A generalizable relationship between mor ... | Vasily Giannakeas · Steven A. Narod |
| A multicentre, randomized pilot trial co ... | Andrew Robinson · Carol Stober · Dean Fe ... |
| A phase Ib, open-label, dose-escalation ... | Vandana G. Abramson · Mafalda Oliveira · ... |
Full Answer
Is there a journal for breast cancer research and treatment?
Breast Cancer Res. Treat. Breast Cancer Research and Treatment is a scientific journal focused on the treatment of and investigations in breast cancer. It is targeted towards a wide audience of clinical researchers, epidemiologists, immunologists, or cell biologists interested in breast cancer.
What research is being done on breast cancer?
Breast Cancer Research. Using a liquid biopsy to test for tumor cells circulating in blood, researchers found that, in women with breast cancer, the presence of these cells could identify women at risk of their cancer returning years later.
Who is the new editor-in-chief of breast cancer research and treatment?
The journal is pleased to announce Dr. Bill Gradishar has been named the new Editor-in-Chief of Breast Cancer Research and Treatment, effective January 1, 2020.
What are the latest advances in breast cancer research?
This page highlights some of the latest research in breast cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies. Breast cancer is one of a few cancers for which an effective screening test, mammography, is available.
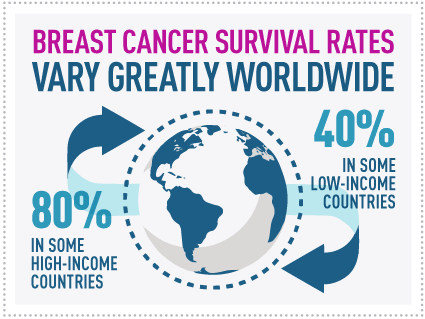
Is Breast Cancer Research and treatment peer-reviewed?
Breast Cancer Research is an international, peer-reviewed online journal, publishing original research, reviews, editorials and reports.
Who is the author of breast cancer Awareness?
Who Started it? Breast Cancer Awareness Month began in 1985 as a partnership between the American Cancer Society and the pharmaceutical division of Imperial Chemical Industries. Betty Ford helped kick off the week-long event, as she was herself a survivor of breast cancer.
What is the leading company in cancer research?
Top 20 pharma companies by oncology sales#CompanyGrowth ($m)1F. Hoffmann-La Roche Ltd5062Celgene Corp16923Novartis AG6684Bristol-Myers Squibb Co2721 more row
Who is doing research on breast cancer?
Within the EDRN, the Breast and Gynecologic Cancers Collaborative Group conducts research on breast and ovarian cancers.
When did breast cancer research start?
Our modern approach to breast cancer treatment and research started forming in the 19th century. Consider these milestones: 1882: William Halsted performed the first radical mastectomy. This surgery will remain the standard operation to treat breast cancer until into the 20th century.
When was the ribbon associated with breast cancer awareness?
Then in 1992, Alexandra Penney, editor-in-chief of Self magazine, wanted to put the magazine's second annual Breast Cancer Awareness Month issue over the top. She did this by creating a ribbon and enlisting the cosmetics giants to distribute them in New York City stores. And thus, the birth of the pink ribbon!
What company is trying to cure cancer?
The U.S. Food and Drug Administration this week approved a Novartis drug as the first ever drug using a new form of cancer treatment called CAR-T cell therapy. Analysts also point to pricing power and potential for acquisition as reasons why they like the handful of stocks of companies working on cancer cures.
Which company has the best oncology pipeline?
Here we discuss four pharma giants, which possess the industry's strongest oncology pipeline portfolios.AstraZeneca AZN.Pfizer PFE.Merck.Bristol-Myers BMY.You can see the complete list of today's Zacks #1 Rank (Strong Buy) stocks here.Others Not Far Behind.Conclusion.Large Cap Pharmaceuticals Industry 5YR % Return.More items...•
What is the best oncology stock?
Ready to invest in the cancer industry? Consider these 9 stocks.Amgen (AMGN)Bristol-Myers Squibb Company (BMY)Five Prime Therapeutics (FPRX)Gilead Sciences (GILD)Novartis AG ADR (NVS)Cardiff Oncology (CRDF)Surface Oncology (SURF)Trillium Therapeutics (TRIL)More items...
What's new in cancer research?
Researchers have identified a protein called CD24 that may be a new target for cancer immunotherapy. The protein is a 'don't eat me' signal that prevents immune cells called macrophages from engulfing and eating cells.
Is there any new treatment for breast cancer?
In March 2019 , the FDA approved atezolizumab (Tecentriq), a new type of drug known as a PD-L1 inhibitor. Atezolizumab is approved for people with locally advanced or metastatic triple-negative breast cancer (TNBC) that can't be surgically removed, or whose tumors express a protein called PD-L1.
How close are they to a cure for breast cancer?
Treatment with a combination of surgery, radiation, and sometimes medication can cure breast cancer in the early stages. The American Cancer Society (ACS) reports that 99 percent of people who receive treatment for breast cancer in the earliest stages live for 5 years or longer after diagnosis.
Publishing options
Breast Cancer Research and Treatment is a Transformative Journal (TJ). Once the article is accepted for publication, authors will have the option to choose how their article is published:
Fees and Funding
Authors who choose to publish open access in Breast Cancer Research and Treatment are required to pay an article-processing charge.
Creative Commons licences
Open access articles in Springer Nature journals are published under Creative Commons licences. These provide an industry-standard framework to support easy re-use of open access material. Under Creative Commons licences, authors retain copyright of their articles.
What is the name of the drug that is used to treat breast cancer?
Alpelisib (Piqray) is approved to be used in combination with hormonal therapy to treat HR-positive and HER2-negative breast cancers that have a mutation in the PIK3CA gene. The drugs above have all been approved to treat metastatic cancer.
What are the mainstays of breast cancer treatment?
The mainstays of breast cancer treatment are surgery, radiation, chemotherapy, hormone therapy, and targeted therapy . But scientists continue to study novel treatments and drugs, along with new combinations of existing treatments.
What is the best treatment for HER2 positive breast cancer?
Neratinib Maleate (Nerlynx) can be used in patients with early-stage HER2-positive breast cancer and can also be used together with capecitabine (Xeloda) in some patients with advanced or metastatic disease. Ado-trastuzumab emtansine (Kadcyla) is an FDA-approved treatment for advanced HER2-positive breast cancer.
What are the three subtypes of breast cancer?
The three main clinical subtypes of breast cancer are: Hormone receptor (HR) positive. HR-positive breast cancers are those that contain the estrogen receptor (ER) and/or progesterone receptor (PR). These cancers grow in response to these hormones and can be treated with hormone therapies.
What is the NCI-sponsored trial?
The study, which included patients with ER-positive, lymph node-negative breast cancer, found that a test that looks at the expression of certain genes can predict which women can safely avoid chemotherapy.
What are the concerns of breast cancer screening?
Two concerns in breast cancer screening, as in all cancer screening, are the potential for diagnosing tumors that will not become life-threatening (overdiagnosis) and the possibility of receiving false-positive test results.
Is breast cancer a screening test?
Breast cancer is one of a few cancers for which an effective screening test, mammography, is available. MRI ( magnetic resonance imaging ), ultrasound, and clinical breast exams are also used to detect breast cancer, but not as routine screening tools. Ongoing studies are looking at ways to enhance current breast cancer screening options.
When biological material is donated for or data is generated as part of a research project, should authors ensure, as part
When biological material is donated for or data is generated as part of a research project authors should ensure, as part of the informed consent procedure, that the participants are made aware what kind of (personal) data will be processed, how it will be used and for what purpose. In case of data acquired via a biobank/biorepository, it is possible they apply a broad consent which allows research participants to consent to a broad range of uses of their data and samples which is regarded by research ethics committees as specific enough to be considered “informed”. However, authors should always check the specific biobank/biorepository policies or any other type of data provider policies (in case of non-bio research) to be sure that this is the case.
When reporting a study that involved human participants, their data or biological material, should authors include a statement that confirm
When reporting a study that involved human participants, their data or biological material, authors should include a statement that confirms that the study was approved (or granted exemption) by the appropriate institutional and/or national research ethics committee (including the name of the ethics committee) and certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. If doubt exists whether the research was conducted in accordance with the 1964 Helsinki Declaration or comparable standards, the authors must explain the reasons for their approach, and demonstrate that an independent ethics committee or institutional review board explicitly approved the doubtful aspects of the study. If a study was granted exemption from requiring ethics approval, this should also be detailed in the manuscript (including the reasons for the exemption).
What does submission of a manuscript mean?
Submission of a manuscript implies: that the work described has not been published before; that it is not under consideration for publication anywhere else; that its publication has been approved by all co-authors, if any, as well as by the responsible authorities – tacitly or explicitly – at the institute where the work has been carried out. The publisher will not be held legally responsible should there be any claims for compensation.
What is Springer Nature?
Springer Nature advocates complete and transparent reporting of biomedical and biological research and research with biological applications. Authors are recommended to adhere to the minimum reporting guidelines hosted by the EQUATOR Network when preparing their manuscript.
What is clinical trial?
The World Health Organization (WHO) definition of a clinical trial is "any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes". The WHO defines health interventions as “A health intervention is an act performed for, with or on behalf of a person or population whose purpose is to assess, improve, maintain, promote or modify health, functioning or health conditions” and a health-related outcome is generally defined as a change in the health of a person or population as a result of an intervention.
What are authors requested to do?
All authors are requested to make sure that all data and materials as well as software application or custom code support their published claims and comply with field standards. Please note that journals may have individual policies on (sharing) research data in concordance with disciplinary norms and expectations.
Do peer reviewers have to check the data availability statement?
Peer reviewers are encouraged to check the manuscript’s Data availability statement, where applicable. They should consider if the authors have complied with the journal’s policy on the availability of research data, and whether reasonable effort has been made to make the data that support the findings of the study available for replication or reuse by other researchers. Peer reviewers are entitled to request access to underlying data (and code) when needed for them to perform their evaluation of a manuscript.
