Monoclonal antibody treatments can be prescribed by health care providers to individuals 12 years of age and older who have been diagnosed with COVID-19 and who have been exposed to someone with COVID-19 and are at high risk for severe illness and hospitalization.
Who can get Regeneron treatment?
Jan 06, 2022 · Who is eligible for monoclonal antibody therapy? Given that COVID-19 vaccination provides strong protection against severe disease and need for hospitalization, monoclonal antibody therapy is an option for certain high-risk patients with COVID-19. THE FDA expanded EUA of two monoclonal antibody treatments to include patients as young as newborns. Criteria for …
How soon should you get monoclonal antibodies?
Aug 20, 2021 · Monoclonal antibody treatment is available to individuals who: Are high risk** for developing severe COVID-19 AND; Have a positive COVID-19 test and have not yet been admitted to the hospital AND; Are 12 years of age or older (and at least 88 pounds) Post-exposure preventive monoclonal antibodies are available to those who have been exposed (consistent …
What are the dangers of monoclonal antibodies?
For people at high risk of getting very sick from COVID-19, monoclonal antibody or antiviral therapy, given early, can greatly reduce the chance of getting COVID-19 and prevent the disease from becoming severe. It also reduces the chance of needing to be in the hospital. The treatment can also shorten how long COVID-19 symptoms last..
Can everyone get Regeneron?
1-877-332-6585. If you are at risk for serious COVID-19 and you have tested positive for COVID-19 or have been in close contact with someone who has tested positive, you may want to consider a monoclonal antibody (mAb) treatment. You may qualify for a mAb treatment ( sotrovimab or bebtelovimab) for this promising COVID-19 treatment depending on your age, health history, …

What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
How do monoclonal antibodies work against COVID-19?
Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
Can I get the COVID-19 vaccine if I was treated with monoclonal antibodies or convalescent plasma?
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
What is a monoclonal antibody?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells.Mar 31, 2022
Should you still get the COVID-19 vaccine if you were treated with monoclonal antibodies?
If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, there is no need to delay getting a COVID-19 vaccine.Feb 17, 2022
What medication is not recommended before vaccinations for COVID-19?
It is not recommended you take over-the-counter medicine – such as ibuprofen, aspirin, or acetaminophen – before vaccination for the purpose of trying to prevent vaccine-related side effects. It is not known how these medications might affect how well the vaccine works.
Who should not take the Pfizer-BioNTech COVID-19 vaccine?
If you have had a severe allergic reaction to any ingredient in the Pfizer-BioNTech COVID-19 vaccine (such as polyethylene glycol), you should not get this vaccine. If you had a severe allergic reaction after getting a dose of the Pfizer-BioNTech COVID-19 vaccine, you should not get another dose of an mRNA vaccine.
What are the contraindications to the COVID-19 vaccine?
Contraindications to COVID-19 vaccination include: Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a component of the COVID-19 vaccine. Known diagnosed allergy to a component of the COVID-19 vaccine (see Appendix C for a list of vaccine components).
What is the first drug that was approved by the FDA to treat COVID-19?
Remdesivir is the first drug approved by the FDA for treatment of hospitalized COVID patients over the age of 12.Jan 25, 2022
Which drug is approved by FDA to treat COVID-19?
Veklury (Remdesivir) is an antiviral drug approved for use in adults and pediatric patients [12 years of age and older and weighing at least 40 kilograms (about 88 pounds)] for the treatment of COVID-19 requiring hospitalization.Mar 31, 2022
How many types of COVID-19 vaccines are available in the US?
Three COVID-19 vaccines are authorized or approved for use in the United States to prevent COVID-19. Pfizer-BioNTech or Moderna (COVID-19 mRNA vaccines) are preferred. You may get Johnson & Johnson's Janssen COVID-19 vaccine in some situations.
What is the purpose of monoclonal antibody therapy?
The goal of this therapy is to help prevent hospitalizations, reduce viral loads and lessen symptom severity.
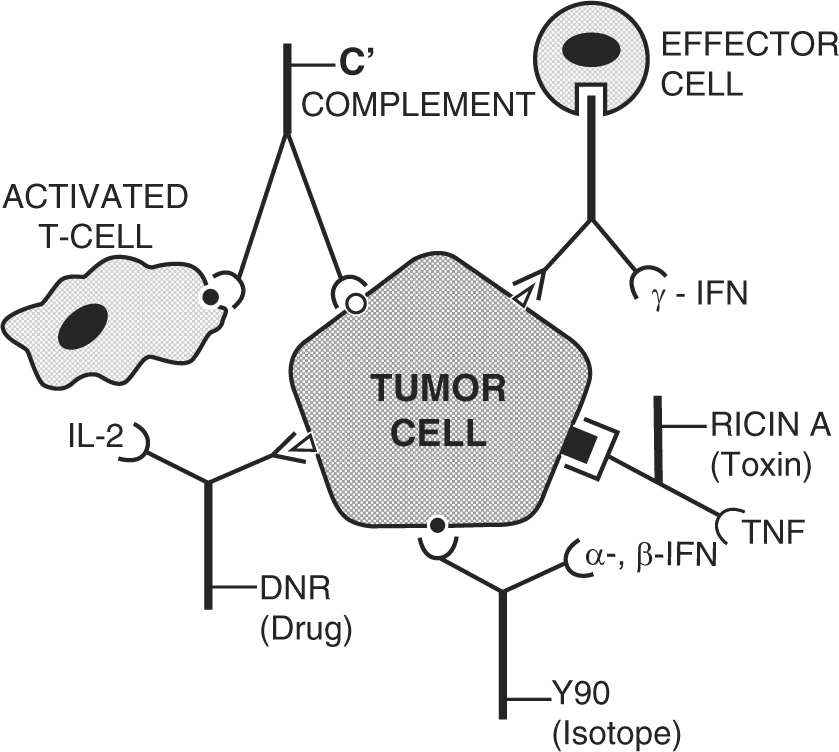
What are monoclonal antibodies?
However, monoclonal antibodies are mass-produced in a laboratory and are designed to recognize a specific component of this virus — the spike protein on its outer shell .
What antibodies interfere with the virus?
By targeting the spike protein, these specific antibodies interfere with the virus' ability to attach and gain entry into human cells. The two monoclonal antibody therapies currently available are the bamlanivimab and a combination of the casirivimab and imdevimab.
How long should you wait to get a second shot?
If you already received the first dose of vaccine before monoclonal antibody therapy, current CDC guidelines recommend you wait 90 days before receiving the second dose. Categories: Tips to Live By. Tags: Coronavirus, Infectious Disease.
What are the high risk people?
Those who are at high risk include people who: Are 65 years of age or older. Are at least 55 years of age and have heart disease, hypertension or a chronic respiratory disease such as COPD. Have a BMI above 35. Have chronic kidney disease.
What antibody is used to block the virus?
Monoclonal antibodies against COVID-19 attach to the virus to block it from entering human cells. The monoclonal antibody protein also “marks” the virus to be broken down by the immune system and cleared from the body.
What is the function of antibodies?
Antibodies are proteins that exist in our bodies as part of our immune system to recognize and defend against harmful viruses and bacteria. Monoclonal antibodies are made in a laboratory and designed to target a specific virus or bacteria.
Can monoclonal antibodies cause nausea?
Most people tolerate monoclonal antibody infusions very well. Some people may experience infusion-related side effects, such as nausea and dizziness, that are short-lived and go away on their own. As with any medication, there is the potential for mild or more severe allergic reactions, which are uncommon.
WHAT IS A MONOCLONAL ANTIBODY?
Your body naturally makes antibodies to fight infection. However, your body may not have antibodies designed to recognize a novel (or new) virus like SARS-CoV-2, the virus that causes COVID-19.
How Can I Get Monoclonal Antibodies?
To receive a mAb you should be referred for treatment by your healthcare professional and directed to available infusion locations. If you do not have a healthcare provider, call the Combat COVID Monoclonal Antibodies Call Center at 1-877-332-6585 to find out who to talk with about your symptoms and treatment.
WHAT IF I DO NOT QUALIFY FOR MONOCLONAL ANTIBODY TREATMENT?
Your healthcare professional may decide you do not qualify for mAb treatment. There could be several reasons for this. You may not meet all eligibility criteria or you may have an underlying health condition that disqualifies you for mAb treatment.
WHAT CAN I EXPECT FROM TREATMENT (INFUSION)?
The mAb treatment is usually offered at an infusion center because the treatment is given through an intravenous (IV) infusion or shots. Depending on the mAb treatment you receive, the whole process takes about 1-3 hours, depending on the treatment..
CAN MONOCLONAL ANTIBODY TREATMENT MAKE ME SICK?
Antibody treatments do not contain any live SARS-CoV-2, so there is no risk you will get COVID-19 from mAb treatment. However, the antibody treatment may have side effects: