Common question
Is there a drug treatment for COVID-19?The U.S. Food and Drug Administration has approved one drug treatment for COVID-19 and has authorized others for emergency use during this public health emergency. In addition, many more therapies are being tested in clinical trials to evaluate whether they are safe and effective in combating COVID-19.Jan 27, 2022
What is the first drug that was approved by the FDA to treat COVID-19?
What medication can I take to reduce the symptoms of COVID-19?
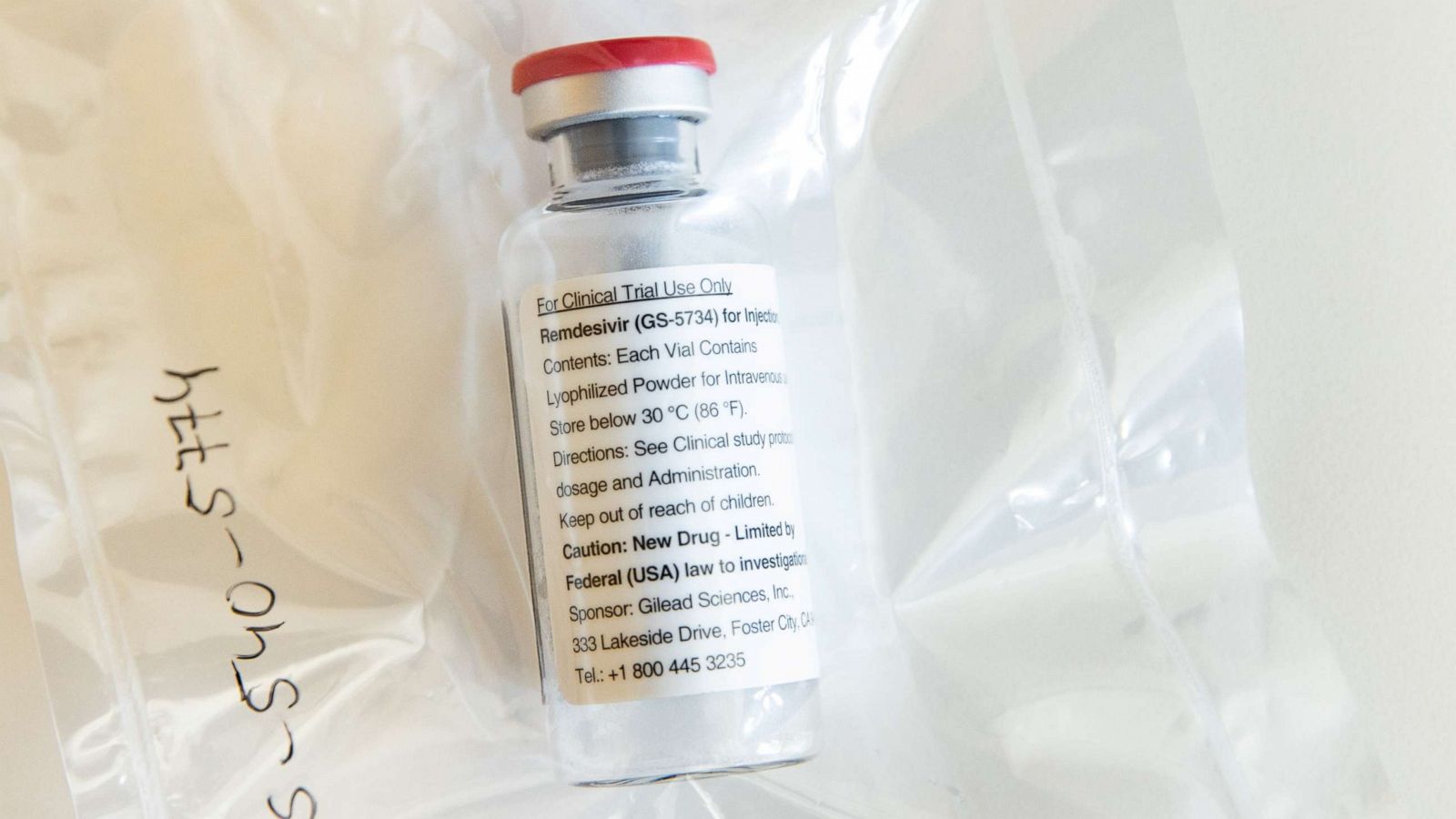
Is Remdesivir approved for treatment of COVID-19?
Is Remdesivir approved in Europe for treatment of COVID-19?
What should I do if COVID-19 symptoms are mild enough and I can recover at home?
• Rest. It can make you feel better and may speed your recovery.
• Stay home. Don't go to work, school, or public places.
• Drink fluids. You lose more water when you're sick. Dehydration can make symptoms worse and cause other health problems.
• Monitor. If your symptoms get worse, call your doctor right away.
Should you take cold medications if you have COVID-19 without symptoms?
If you have COVID-19 but don't have symptoms, don't take cold medications, acetaminophen (Tylenol), or over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen (Advil®) and naproxen (Aleve®). These medications may hide the symptoms of COVID-19.
How does Remdesivir injection work to treat COVID-19?
What are the side effects of Remdesivir?
Remdesivir may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
• nausea
• constipation
• pain, bleeding, bruising of the skin, soreness, or swelling near the place where the medication was injected
Is there an emergency use authorization of Paxlovid for COVID-19 in the US?
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the emergency use of the unapproved product PAXLOVID for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kg)
What is Remdesivir?
What is the Novavax vaccine?
Is Paxlovid a prescription drug?
Out-Of-Hospital Treatment Options For Covid-19
- Oral Antiviral Treatments
The FDA authorized two oral antivirals, Pfizer's Paxlovid and Merck's molnupiravir, for the treatment of COVID-19 in certain patients. - Monoclonal Antibody Treatments
COVID-19 monoclonal antibody therapeutics (mAb)are available for people ages 12 years or older who: 1. Have tested positive for COVID-19 and have had symptoms for 10 days or less 2. Are at high risk of becoming seriously ill, including those who have been recently exposed to someone …
Hospital Treatments For Covid-19
- There are treatments for hospitalized patients with severe cases of COVID-19 that have been approved or authorized for emergency use by the Food and Drug Administration (FDA). 1. Remdesiviris an antiviral drug approved by the FDA for the treatment of COVID-19 in hospitalized adults and hospitalized pediatric patients at least 12 years of age. It works by stopping SARS-Co…
Ensuring The Safety and Effectiveness of Treatments
- After a public health emergencywas declared for the COVID-19 pandemic, it was determined that the Food and Drug Administration (FDA) could authorize the emergency use of tests, treatments, and vaccines to reduce suffering, loss of life and restore the health and security of our country. 1. FDA has approved the use of one anitviral drug Veklury (remdesivir) to treat COVID-19. FDA has …