When water contains high amounts of magnesium hardness, split treatment may be used. Approximately 80 percent of the water is treated with excess lime to remove magnesium at a pH above 11, after which it is blended with 20 percent of the source water. Split treatment can reduce the amount of carbon dioxide required to re-carbonate the water as well as offer a savings in lime feed.
What is lime used for in water treatment?
In terms of annual tonnage, lime ranks first among chemicals used in the treatment of potable and industrial water supplies. Lime is used by many municipalities to improve water quality, especially for water softening and arsenic removal.
When is the best time to apply lime to your lawn?
You can apply lime anytime throughout the year, but fall in the most beneficial time. Using lime in the fall allows the lime to break down over the winter months and get the soil better for springtime growth. After applying lime, make sure to water the lawn to allow the lime to contact the soil. How Often Should I Apply Lawn Lime Treatment?
What is the hardness of excess lime?
The two-stage, excess lime softening process provides the most complete softening. It is capable of removing calcium and magnesium carbonate and noncarbonate hardness, down to the solubility limits of about 30 to 40 mg/L of calcium hardness and about 10 mg/L of magnesium hardness.
What is lime treatment for lawns?
A lawn care treatment often overlooked is a lime application — a lawn care treatment that helps balance your soil’s pH levels. Over time, your soil can become acidic, which is not optimal for healthy grass to grow in specific environments.

What happens when excess lime is added to water?
When lime is added to water, carbon dioxide present in the water is converted to calcium carbonate if enough lime is added. Adding more lime, the calcium bicarbonate will be precipitated as calcium carbonate. To remove calcium and magnesium bicarbonate, an excess of lime must be used.
What is the use of lime required in the excess lime treatment?
8. What is the dose of lime required in the excess lime treatment? Explanation: The dose of lime used in the excess lime treatment is 10-20ppm and the excess lime can be removed by the process of re-carbonation. 9.
What is excess lime?
Lime is the common name for Ca(OH)2. It is a source of calcium and alkalinity. Excess lime (lb/bbl) in mud is used as an alkalinity buffer. Excess lime (lb/bbl) = 0.26 [Pm - (Fw x Pf)], where Fw=% of water volume /100.
Why does magnesium bicarbonate require double amount of lime for softening?
Magnesium carbonate required double the amount of lime for softening because: MgCo₃ is alkaline in nature, lime is used to neutralize the effect of the base. MgCo₃ is an ionic in nature. Ionic bonds need more lime to break the bond and neutralize the effect.
Why is excess lime added during the lime soda softening process?
EXCESS LIME TREATMENT To reduce magnesium hardness, more lime must be added to the water. Extra lime will raise pH above 10.6 to help magnesium hydroxide precipitate out of the water.
What is the purpose of lime water in an experiment?
Limewater can be used to detect carbon dioxide. If carbon dioxide is bubbled through limewater then it turns from clear to cloudy/milky in colour. This is why limewater used in a simple respirometer can show that more carbon dioxide is present in exhaled air compared to inhaled air.
How soon can cattle graze after lime?
Grass can be grazed as soon as the lime has been washed off the leaves by rain. If the lime advice for grassland exceeds 7.5 t/ha ;initially only this amount should be applied, and the remainder applied after two years.
Can you put down too much lime?
Using too much lime on your lawn will remove the acidity from the soil, but it will also make it too alkaline for your grass to thrive. This will cause yellowing grass that is also not able to absorb vital moisture and nutrients from the soil around it.
How do you fix too much lime in soil?
If you add too much lime to your soil and make the pH too high, there is a way to fix it. You can add sulfur to your soil to lower the pH, making it more acidic. This can be done with elemental sulfur or ammonium sulfate, but be careful!
Does lime softening reduce TDS?
In lime softening, there is a substantial reduction in total dissolved solids (TDS). In ion exchange softening (sometimes referred to as zeolite softening), there is no significant change in the level of TDS. Lime softening can also be used to remove iron, manganese, radium and arsenic from water.
How does lime soda treatment remove permanent hardness?
Lime soda process: In lime-soda process, hard water is treated with lime (CaO or Ca (OH)2) firstly, after that with soda. In this process, the hardness is removed by sedimentation as calcium carbonate or magnesium hydroxide.
How does calcium bicarbonate affect pH?
Bicarbonate tends to increase pH over time. It tends to precipitate out positively charged ions, specifically calcium, iron, magnesium.
How long does lime softening take?
Lime sludge. The softening process usually requires two sedimentation basins, each with a detention time of 1.5 to 3 hours, to deal with the large quantities of sludge.
What is the chemical reaction of lime?
The goal of all of these reactions is to change the calcium and magnesium compounds in water into calcium carbonate and magnesium hydroxide. These are the least soluble calcium and magnesium compounds and thus will settle out of the water at the lowest concentrations. For example, calcium carbonate (which is essentially the same as limestone) will settle out of water at concentrations greater than 40 mg/L.#N#In order to produce calcium carbonate and magnesium hydroxide, the pH of the water must be raised by the addition of lime. Calcium compounds in water will be removed at a pH of about 9.0 to 9.5 while magnesium compounds require a pH of 10.0 to 10.5. When soda ash is used to remove noncarbonate hardness, an even higher pH is required - 10.0 to 10.5 for calcium compounds and 11.0 to 11.5 for magnesium compounds.
Why is my lime soda ash soft?
If softening problems are discovered, the cause usually lies in either chemical feeder malfunctions or source water quality changes. A variety of water characteristics can influence lime-soda ash softening: Water hardness will determine the quantity of chemicals which must be added to soften the water.
What is the difference between lime and soda ash?
In both calculation methods, lime and soda ash dosages depends on carbonate and non-carbonate hardness in the water. Lime is used to remove carbonate harness, and both lime and soda ash are used to remove non-carbonate hardness.
What is the pH of lime ash?
Solids content in the sludge range from 5 to 30 total solids with a pH greater than 10.5.
What is the most common process used to reduce pH?
Recarbonation. After adding lime and/or soda ash, treated water will generally have a pH greater than 10. It is necessary to lower the pH to stabilize the water and prevent deposition of carbonate scale on filter sand and distribution piping. Recarbonation is the most common process used to reduce pH.
What can replace soda ash?
Caustic soda (NaOH), also known as sodium hydroxide, can replace soda ash and some of the lime in the treatment process. The treatment process using caustic soda follows the same steps as that of lime-soda ash softening. First, carbon dioxide reacts with the caustic soda to make sodium carbonate and water.
Is lime easier to apply than powder?
However, most gardeners find that pellet forms are easier to apply than powders.
Can you put lime on a wet lawn?
Don’t spread lime on a dry, wilted lawn or a soggy, wet lawn. Don’t lime during frosty weather. If you haven’t planted grass seed yet, apply lime to the soil just before you plant. ...
Do you need to lime your lawn?
Here’s a hint that may help you determine if you need lime lawn treatment: If you live in a dry, desert climate, there’s a chance your soil is alkaline and you may not need to lime your lawn grass. If you live a rainy area where acid-loving plants such as rhododendrons and camellias thrive, your soil is likely acidic and may benefit ...
Why is lime used in water treatment?
In some water-treatment plants, alum sludge is treated with lime to facilitate sludge thickening on pressure filters.
What is lime used for?
Lime is used by many municipalities to improve water quality, especially for water softening and arsenic removal.
How does lime help with water?
Lime is also used to combat "red water" by neutralizing the acid water, thereby reducing corrosion of pipes and mains from acid waters. The corrosive waters contain excessive amounts of carbon dioxide. Lime precipitates the CO 2 to form calcium carbonate, which provides a protective coating on the inside of water mains.
How does lime affect pathogens?
Effect on Pathogen Growth - By raising the pH of water to 10.5-11 through the addition of lime and retaining the water in contact with lime for 24-72 hours, lime controls the environment required for the growth of bacteria and certain viruses.
Why is lime used in phenolic water?
This application of lime is utilized where "phenolic water" exists, because chlorine treatment tends to produce unpalatable water due to the presence of phenol. This process, called 'excess alkalinity treatment', also removes most heavy metals.
What are the limitations of lime soda softening?
Limitations of the lime-soda softening process include: An inability to remove all hardness. A high degree of operator control must be exercised for maximum efficiency in cost, hardness removal, and water stability. Color removal may be complicated by the softening process because of high pH levels.
What is the best way to soften lime ash?
An alternative method to the lime-soda ash softening process is the use of sodium hydroxide (NaOH), which is called caustic soda.
How hard is lime water?
The minimum hardness that can be achieved by lime softening-soda ash processes is around 30 to 40 mg/L as calcium carbonate.
Is lime ash hardened water?
Lime-soda ash softened water is usually supersaturated with calcium carbonate. The degree of instability and excess calcium carbonate depends on the degree to which the water is softened. Calcium carbonate hardness is removed at a lower pH than magnesium carbonate hardness.
How does lime soda soften?
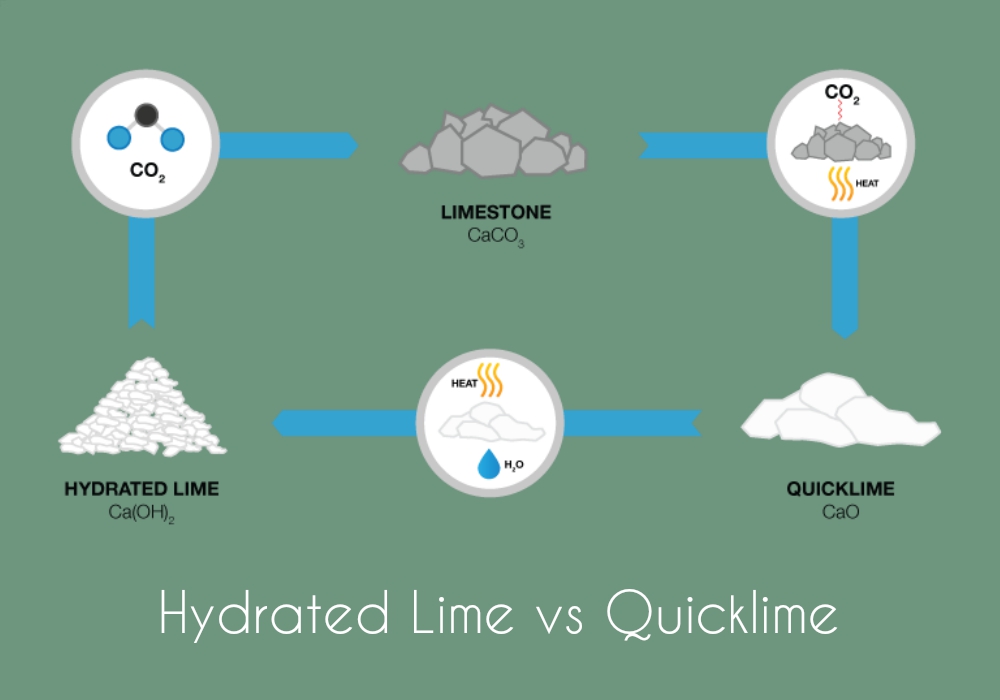
Lime soda softening uses addition of lime and soda ash to remove Ca ++ and Mg ++ ions, bringing their concentration down to an acceptable level. The lime may be in the form of quicklime (CaO) or hydrated lime (Ca (OH) 2 ), also called slaked lime. Soda Ash is Na 2 CO 3 . Calcium ions are removed be bringing the pH level up enough to convert Ca (HCO 3) 2 to CaCO 3, which is relatively insoluble in water and precipitates out down to a residual level of about 30 to 40 mg/L. Magnesium is precipitated out as Mg (OH) 2 . Some lime soda softening processes require addition of carbon dioxide (recarbonation) at one or more points in the process to reduce the pH.
What is the hardness of lime soda?
For lime soda water softening chemistry it is also necessary to have knowledge about the anions in the water. Hardness due to Ca ++ or Mg ++ together with carbonate (CO 3=) or bicarbonate (HCO 3–) is called carbonate hardness . At typical drinking water pH, the anion for carbonate hardness is almost completely bicarbonate.