Methods
- Lime softening. Lime softening is the process in which lime is added to hard water to make it softer. It has several...
- Chelating agents. See templates for discussion to help reach a consensus. ... This section does not cite any sources.
- Washing soda method. In this method, water is treated with a calculated amount of washing soda (Na 2 CO 3 ), which...
What is the best treatment for hard water?
Feb 02, 2022 · The best hard water solution allows water to pass through the double spiral flow form, causing an implosion in the water. This process will soften minerals such as calcium and magnesium in hard water. Here are a few methods to soften hard water. There are several ways to soften hard water you can try. Some of the common methods are:
How can I soften my water at home?
Apr 02, 2022 · Method 3: Boil Hard Water. Method Rating: 6/10. ★★★★★★★★★★. Boiling hard water removes many of the hardness minerals that cause scale. That’s why you might notice that the inside of your stovetop kettle or coffeepot has floating chunks of limescale. This method is simple enough: just boil the water you want to soften ...
How to soften water without a water softener?
Nov 24, 2017 · The only real way to soften hard water is to remove the calcium and magnesium minerals that make the water hard. Only ion exchange softeners and some water filters do this. Water filters; Some common water filters that will do the job of producing soft water: caron resin filters, reverse osmosis units, water distillers. These are only available in a single outlet, not …
How to soften hard water in your home?
Magnetic water softening claims to reduce the scale build up and the affects of hard water. The magnetic devises are very inexpensive compared to other methods of softening water. Disadvantages The degree of efficiency is constantly changing. The magnetic field exists only in the immediate vicinity of the

What are the methods of softening hard water?
How to Soften Hard Water. 4 Best Methods For Softening WaterIon-Exchange Water Softening.Salt-Free Water Conditioning: Template Assisted Crystallization (TAC) / Nucleation Assisted Crystallization (NAC)Salt-Free Water Conditioning: Reverse Osmosis (RO)Salt-Free Water Conditioning: Chelation.Jan 17, 2021
What is the best way to treat hard water?
The most common way to treat hard water is with a Water Softener. This is a water filtration system that filters out the hard water minerals in your water. Was the water travels into the filter, it passes through a bed of resin that traps the calcium and magnesium, which are then replaced with sodium ions.Jan 14, 2021
How do you soften water naturally?
One of the most common ways to soften hard water is through the use of salt. Most people who are curious about how to soften hard water naturally will lean towards the use of an ion-exchange water softener. Salt plays a critical role in the functionality of these water softening systems.
How do you soften hard water without a water softener?
Install an ion-exchange filter to your kitchen faucet or use a water pitcher filter. Install a showerhead with a built-in shower filter: Softened shower water has many benefits for your skin and hair health. Use a moisturizer after showering to keep your skin from drying out due to hard water.Aug 4, 2020
What is magnetic water softening?
Magnetic water softening claims to reduce the scale build up and the affects of hard water. The magnetic devises are very inexpensive compared to other methods of softening water.
How does NaturSoft media work?
The NaturSoft media has calcium carbonate crystal structures on its surface that will attract excess dissolved hardness and remove it from solution by integrating it into the crystal structures on the media. This results in the crystals on the media surface to grow larger. The movement of water and friction among the individual media granules rubbing against each other will cause fragments of these newly grown crystal structures to be fragmented off the media and released into the passing water. Those free flowing calcium carbonate crystals then continue to travel through a plumbing system as suspended particles where they perform the same role as the media in the NaturSoft system itself, i.e. acting as seed crystals further buffering the effects of any changes in the scale potential of the water downstream by absorbing excess mineral into their structure and themselves spawning the creation of additional micro crystals.
Is salt water safe for drinking?
Softened water from a salt-based water softener is not recommended for drinking, watering houseplants, lawns and gardens due to its sodium content. There are many health risks associated with sodium intake. During the softening process sodium is released from the exchange media into the output water. For every grain of hardness removed from water, 8mg/1 (ppm) of sodium is added. People on restricted sodium intake diets should account for increased levels of sodium in softened water. Your family physician should be consulted. Sodium intake from softened water can be avoided by leaving one kitchen tap un-softened from drinking and cooking. Water used in recharging a water softener may over load or reduce the effectiveness of small septic or sewer systems. Softened water is not recommended for small appliances such as steam irons or evaporative coolers. There are additional cost and maintenance required. Salt-based softeners require that salt be added to the system on a regular basis based upon the hardness of the water. Cost of salt is approximately $5 to $7 per 40-pound bag depending on the form
How does reverse osmosis work?
Reverse osmosis involves the reversal of flow through a membrane from a high salinity, or concentrated, solution to the high purity, or permeate , stream on the opposite side of the membrane. Pressure is used as the driving force for the separation. The applied pressure must be in excess of the osmotic pressure of the dissolved contaminants to allow flow across the membrane. For example, the membrane may allow passage of water molecules, but blocks molecules of dissolved salt. The membrane retains unwanted molecules while the ultra-pure water continues on for use or further treatment. This process takes any unwanted molecules retained by the membrane and sweeps them away to the drain.
Is reverse osmosis water safe for plants?
Reverse osmosis strips the essential minerals from the water therefore it is not suitable for plants, animals, humans or cooking (according to the World Health Organization). Reverse osmosis water is hard on plumbing and fixtures, due to the non-mineral content in the water. Reverse osmosis only recovers between 5% and 15% of the water entering the system; the rest of the water is wasted. The system requires a connection to a drain for wastewater. The process is relatively slow and requires a storage tank. Low water pressure and high temperatures adversely affect the production of water. A booster pump may need to be installed for low-pressure situations. The storage unit for treated water will support bacteria growth unless regularly disinfected. Costly membranes will need to be replaced periodically.
Why is hard water treated?
The primary purpose of hard water softening is to prevent the precipitation and buildup of hard water minerals in equipment and piping.
What is the purpose of hard water softening?
The primary purpose of hard water softening is to prevent the precipitation and buildup of hard water minerals in equipment and piping. Reduction or elimination of hard water scaling can be performed using physical water treatment equipment, or, in limited circumstances, using chemical additives.
How many tanks does a water softener have?
A water softener typically consists of two tanks, a larger one into which rock or pellet salt is added and a smaller tank containing the ion exchange resin through which the hard water passes. A control valve fixed atop the resin tank of the industrial water softener causes the system to recharge or regenerate based on passage ...
Is water a solvent?
Water is a universal solvent. Soft rainwater picks up naturally occurring dissolved minerals, including calcium and magnesium carbonates, as it passes over rocks and through soil, which begins to make it hard. Most source water contains some amount of hardness.
What is permanent hardness?
Permanent Hardness. Water that contains other anions such as chloride or sulfate cannot be softened by boiling and are said to be “permanently” hard. These must be softened by other methods, including lime soda or, more frequently, ion exchange softening described below.
Is calcium carbonate soluble in water?
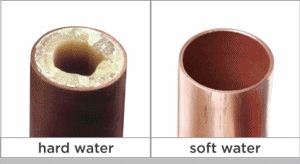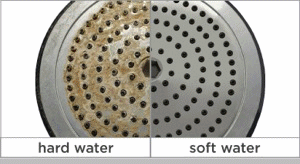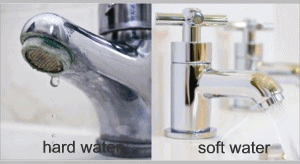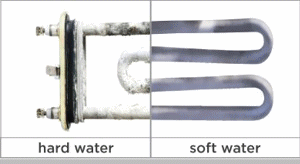
Calcium carbonate is moderately soluble in water and will come out of solution (i. e., form a precipitate) in the form of a hard scale when its concentration in water exceeds its solubility constant. This tendency may cause build-up in hot and cold-water pipes, water heaters, boiler tubes, cooling towers and any other surfaces it contacts. It also reacts with soap and detergent forming a precipitate in the form of a “scum” which is evident as spotting on glasses and silverware and as “bathtub ring.” The buildup in boilers can interfere with the transfer of heat and can even lead to boiler tube failure.
What are the disadvantages of ion exchange resin?
Disadvantages include the need to dispose of wastewater high in salinity and the fact that water treated with an ion exchange water softener has sodium added.
Why does my kitchen faucet smell?
If you notice that your faucets have a chalky white buildup or your dishware sometimes gets spots on it, your home might have hard water. Hard water usually contains a high concentration of minerals like calcium or magnesium, which can cause a funny taste or smell.
What is the best way to clean clothes?
When you throw a load into your washing machine, sprinkle washing soda over your clothes before you start the washer. Washing soda also helps lift dirt and grime from fabric, so it can lead to cleaner clothes. The carbonate ions from washing soda react with the calcium and magnesium ions that are in hard water.
How to get rid of chlorine smell in shower?
1. It will remove chlorine and lead from your water. Shower filters are also effective at neutralizing unpleasant smells, and they are specially designed to work with high temperatures and flow rates. You can find shower head filters at hardware stores, home centers, and online marketplaces.
Does boiling water remove calcium?
The sediment will most likely be white and chalky. That’s the calcium leaving the water. Boiling doesn’t get rid of all the impurities in hard water, only the calcium. However, calcium is usually what causes the unpleasant smell and taste, so it can greatly improve your water.
What is the best way to filter hard water?
Peat moss naturally filters hard water and purifies it. Purchase a layer of peat moss from an aquarium store and place it at the bottom of your tank. Over time, the humic acid and the tanning agents in the moss will soften the water and filter it.
How to remove calcium from hard water?
Boiling removes calcium from hard water. Fill a clean pot or kettle with water and place it on a stove burner set to high. Allow the water to boil for a few minutes, then turn off the heat. Let the water cool, then use a spoon to scoop the sediment off the top of the water before transferring it to a clean container.
Does baking soda soften water?
Some foods become tough and rubbery when cooked in hard water. While baking soda can’t soften water completely, it will help alter the pH level to a more natural state. Fill up a pot of water and add 1 tsp (5.6 g) of baking soda when you’re cooking dried beans and peas for a better taste and texture.
What is the reaction of carbon dioxide and water?
Carbon dioxide reacts with water to form carbonic acid (1) which at ordinary environmental pH exists mostly as bicarbonate ion (2). Microscopic marine organisms take this up as carbonate (4) to form calcite skeletons which, over millions of years, have built up extensive limestone deposits. Groundwaters, made slightly acidic by CO 2 (both ...
Can water be boiled to remove ions?
Waters than contain other anions such as chloride or sulfate cannot be remediated by boiling, and are said to be "permanently" hard. The only practical treatment is to remove all the ions, normally by the method described below.
What is temporary hardness?
Temporary hardness. This refers to hardness whose effects can be removed by boiling the water in an open container. Such waters have usually percolated though limestone formations and contain bicarbonate HCO 3– along with small amounts of carbonate CO 32– as the principal negative ions. Boiling the water promotes the reaction.
What is the process of water softening?
Most conventional water-softening devices depend on a process known as ion-exchange in which "hardness" ions trade places with sodium and chloride ions that are loosely bound to an ion-exchange resin or a zeolite (many zeolite minerals occur in nature, but specialized ones are often made artificially.)