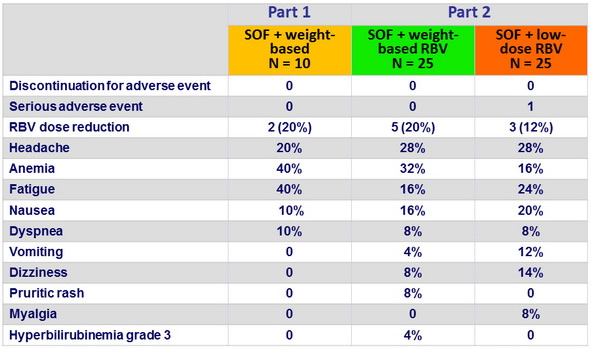
The first two direct-acting antiviral agents (DAAs) for genotype-1 HCV (HCV-1) were approved in Australia in 2012, and have been Pharmaceutical Benefits Scheme listed from 1 April 2013. These two drugs, telaprevir and boceprevir, are both inhibitors of the HCV NS3 protease, which is necessary for viral replication. They are specific for HCV-1.
Is Australia leading the way in managing hepatitis C?
· The following DAA medicines are currently prescribed in Australia to cure hepatitis C: Epclusa® (sofosbuvir + velpatasvir) Maviret® (glecaprevir/pibrentasvir) Harvoni® (sofosbuvir + ledipasvir) VOSEVI® (sofosbuvir + velpatasvir + voxilaprevir). Only used if previous DAA treatment has failed. 4
Can Pharmacists dispense DAA medications for HCV in Australia?
The integration of HCV therapy with addiction therapy in opioid substitution treatment centres represents an opportunity to enhance HCV treatment uptake. Successful Australian models have been described, demonstrating feasibility and cost-effectiveness.
What are the treatment options for hepatitis C (HCV)?
Treatment. Of the five main DAAs being offered in Australia, three can treat all genotypes in as little as 8 to12 weeks. These three pangenotypic drugs are: Epclusa (sofosbuvir + velpatasvir), Maviret (glecaprevir + pibrentasvir) and; Vosevi (sofosbuvir + velpatasvir + voxilaprevir).
Can General Practitioners (GP) prescribe HCV treatment?
· The first line cures for hep C currently are: Epclusa (all genotypes and for 12 weeks) Maviret (all genotypes and for 8 weeks) Who Can Access The Cures? Hep C cures are now available to everyone in Australia who has hep C.* The national and state governments want everyone with hep C to be cured, including prisoners and people who inject drugs.
What types of treatments are available for HCV?
Hepatitis C is treated using direct-acting antiviral (DAA) tablets. DAA tablets are the safest and most effective medicines for treating hepatitis C. They're highly effective at clearing the infection in more than 90% of people. The tablets are taken for 8 to 12 weeks.
How much does Epclusa cost in Australia?
The drug Epclusa® – a combination of sofosbuvir 400mg and velpatasvir 100mg – is the first of the direct-acting antiviral treatments effective for all types of the disease. It will cost most patients A$38.80, and A$6.30 for concession card holders.
Is Harvoni still used to treat Hep C?
Important Safety Information. HARVONI is a prescription medicine used to treat adults with chronic (lasting a long time) hepatitis C (Hep C) genotype (GT) 1, 4, 5 or 6 infection with or without cirrhosis (compensated).
What type of medication is used to cure the majority of cases of chronic HCV?
The current treatment of choice for chronic HCV infection is a combination of pegylated interferon alfa and ribavirin.
Is Hep C treatment covered by Medicare?
Medicare covers screenings to detect hepatitis C, often at no cost. Medicare Part D plans must include at least one hepatitis C treatment medication. These prescription drugs are often still expensive if you don't have a low-income subsidy to help pay for them.
When was Epclusa approved Australia?
This product received initial registration on the Australian Register of Therapeutic Goods (ARTG) on 19 December 2016 for the following indication: Epclusa (sofosbuvir/velpatasvir fixed-dose combination) is indicated for the treatment of chronic hepatitis C virus (HCV) infection (genotype 1, 2, 3, 4, 5 or 6) in adults.
Which is better Epclusa vs Harvoni?
Which is more effective: Epclusa or Harvoni? Although both Epclusa and Harvoni are effective for treating hepatitis C, Epclusa may cure a greater percentage of people than Harvoni does. Epclusa was approved in 2016 and was the first medication approved that was effective at treating all six hepatitis C genotypes.
What is the difference between Mavyret and Harvoni?
Mavyret is approved to treat chronic HCV genotypes 1, 2, 3, 4, 5 or 6 infection in patients without cirrhosis or with compensated cirrhosis (Child-Pugh A), whereas Harvoni is only approved to treat genotypes 1, 4, 5, or 6. In addition, Mavyret is typically given for only 8 weeks, whereas Harvoni is given for 12 weeks.
What is the generic name for Harvoni?
Generic Name: ledipasvir-sofosbuvir This medication is a combination of ledipasvir and sofosbuvir and is used to treat chronic (long-lasting) hepatitis C, a viral infection of the liver. It may sometimes be used with another antiviral medication (ribavirin).
What is the newest treatment for hep C?
Recent advances in antiviral treatment have led to the development of new highly effective drugs for the treatment of all types of hepatitis C. The new hepatitis C treatments are sofosbuvir with ledipasvir (Harvoni); sofosbuvir (Sovaldi); daclatasvir (Daklinza); and ribavirin (Ibavyr).
What is the first line of treatment in hepatitis?
Currently, pegylated interferon alfa (PEG-IFN-a), entecavir (ETV), and tenofovir disoproxil fumarate (TDF) are the first-line agents in the treatment of hepatitis B disease.
Is hep C treatment like chemo?
But Bacon says hepatitis C treatment can have side effects "that are akin to what patients experience when they receive cancer chemotherapy." That includes temporary hair loss. The peginterferon-ribavirin combination is "sometimes loosely called chemotherapy," says Bacon.
Who can prescribe HCV?
GPs who are not experienced in the treatment of HCV are eligible to prescribe the new HCV medicines provided this is done in consultation with an experienced gastroenterologist, hepatologist or infectious diseases physician.
What is the TGA test for HCV?
In May 2020, a point-of-care assay for HCV RNA was approved by the Australian Therapeutic Goods Administration (TGA). The Xpert® HCV viral load assay (Cepheid) measures HCV RNA from a finger-prick blood sample (100 m L) and provides a real-time result in less than 60 minutes.
What is tertiary care?
Tertiary care clinics led by gastroenterologists, hepatologists or infectious diseases physicians have traditionally been the main sites for HCV clinical referral, assessment and treatment. Tertiary treatment centres should continue to be the main treatment sites for people with chronic HCV infection who have cirrhosis, ...
Can a general practitioner prescribe under PBS?
This means that general practitioners are eligible to prescribe under the PBS in consultation with one of these specialists. “In consultation with” means that a GP must consult with one of the specified specialists by phone, fax, mail, email or videoconference to meet the prescriber eligibility requirements. Once GPs are experienced in treating ...
Why has the health care system failed to deal with the HCV epidemic?
The reasons why the health care system has previously failed to effectively deal with the HCV epidemic are multifactorial and include the toxicity of IFN-based antiviral therapy, insufficient linkage to tertiary hospital-based care for socially marginalised individuals, capacity constraints in tertiary care and a lack of alternative models of care. The introduction of new DAA regimens was a major advance for HCV therapy. [8] Their high efficacy, short duration and excellent tolerability mean that most people are now suitable for treatment, most people who start treatment will be cured, and treatment is possible in the community as well as in specialist centres.
Is DAA a mental health treatment?
DAA treatment is not associated with the mental health side effects associated with IF N-based therapy. It is important to raise awareness of HCV testing and treatment among professionals and patients in the mental health community. HCV testing and treatment should be incorporated into models of care for people with mental illness.
Can hepatitis SA be answered?
Hepatitis SA can answer many of your questions and talk to you about any concerns you have. We can also put you in touch with other people who have been on treatment who can share their experiences. Having a supportive network of friends, family and services could be helpful if you decide to start treatment.
Can over the counter medicine affect your chances of a cure?
If you are taking other medicines including over the counter drugs or herbal remedies, please check with your specialist as there may be harmful interactions which may affect your chance of a cure.
What is the best treatment for hep C?
The new cures for hep C include: 1 Epclusa (all genotypes and for 12 weeks) 2 Harvoni (genotype 1 and for 8 or 12 weeks) 3 Maviret (all genotypes and for 8 weeks) 4 Zepatier (genotypes 1 & 4 and for 12 weeks)
Is hep C cured?
The new cures for hep C are different to the previous treatments that were available before 2016. Now around 95%, or more, of people who take them are cured, even if your hep C has resulted in liver cirrhosis. The new cures for hep C include:
Does hep C help with cirrhosis?
Curing your hep C clears the virus from your body. It reduces liver inflammation and can help reverse fibrosis and even cirrhosis. Live free from the worry of hep C – knowing that you no longer have hep C can help you feel better about yourself. For example, you may no longer feel worried about passing hep C to other people.
Can you test for hep C?
The national and state governments want everyone with hep C to be cured, including prisoners and people who inject drugs. Now is a very good time to consider testing for hep C or speaking to your doctor about the hep C cures.
Is the information we offer in our resources designed to replace professional medical advice?
The information we offer in our resources is not designed to replace professional medical advice. We inform you to provide you with the best possible pathways to accessing treatment from a professional.
How many people in Australia have hepatitis C?
Of an estimated 188,000 people in Australia with chronic hepatitis C, around 85,000 had been treated with direct-acting antiviral therapy by the end of 2019. Importantly, uptake was comparable, if not higher, among high-risk populations including people who currently inject drugs and HIV-infected gay and bisexual men, ...
How long does hepatitis C treatment last?
1 Several regimens have been licensed since 2014 which allow simple, once-daily oral dosing for 8–12 weeks. These regimens have proved to be tolerable and highly efficacious (cure rates of >95%). The listing of direct-acting antiviral regimens on the Pharmaceutical Benefits Scheme (PBS) from March 2016 has transformed the management of hepatitis C in Australia and has provided optimism for the elimination of hepatitis C virus.
What is the third hepatitis C screening?
Third, hepatitis C virus screening (and potential treatment) needs to be integrated into settings with high-risk populations. These include prison entry, on admission to hospital for drug injecting-related conditions, and within drug and alcohol and mental health services.
Can GPs test for hepatitis C?
GPs clearly have a key role in increasing testing for hepatitis C. Taking a non-judgemental approach, they should consider testing patients with elevated liver enzymes but no readily identifiable risk factors. Hepatitis C assessment and treatment monitoring have been simplified with the advent of well-tolerated, highly curative direct-acting antiviral therapy. However, key elements remain including staging of liver disease (hepatic elastography, shear-wave elastography, surrogate biomarkers), evaluation of potential drug–drug interactions and testing for HIV and chronic hepatitis B.
Is Australia a leader in hepatitis C?
Australia is an international leader in its shift to managing hepatitis C in primary care and drug and alcohol services, with most people now prescribed therapy by non-specialists.
When will hepatitis C be eliminated?
The World Health Organization (WHO) has developed ambitious targets for hepatitis C virus elimination by 2030. These include:
Will Australia meet the WHO elimination target?
Australia has clearly laid the foundations to meet the WHO elimination targets by 2030. Although COVID-19 has been a setback in many other areas of public health, we will get to the ‘other side’ hopefully in 2021, and can re-engage our efforts to strive for hepatitis C elimination.
When will Australia eliminate hepatitis C?
A recent report by the Kirby Institute estimated Australia was on track to eliminate hepatitis C by 2026 – four years earlier than the WHO goal.
How much of the antiviral medication is written in community pharmacies?
General practitioners and other non-specialists now write at least half of prescriptions for the new antivirals, with around 80% of treatments dispensed in community pharmacies.
Is the government proactive in treating hepatitis C?
The government has taken such a proactive approach to treating hepatitis C for several reasons.
How to increase hepatitis C testing in Australia?
Our modelling suggests that hepatitis C RNA testing in Australia would need to increase by at least 50% for the WHO elimination targets to be achieved. One approach to increasing the identification of hepatitis C‐exposed people would be to use rapid point‐of‐care antibody tests, reducing the need for multiple appointments to obtain a diagnosis. 18 In Australia, targeted testing programs are being piloted, including rapid point‐of‐care saliva testing for hepatitis C antibodies at needle and syringe program sites. 19 While point‐of‐care antibody and subsequent RNA testing may reduce the number of patient visits required for a diagnosis, the care cascade could be improved further by employing RNA tests as screening tools, particularly as their costs decline. Other methods for improving the care cascade include providing standard on‐site hepatitis C testing in primary and secondary enhanced needle and syringe programs, increasing standard testing in community mental health services, opt‐out testing for prisoners, and introducing mandatory reporting of hepatitis C testing as key performance indicators for opioid substitution therapy clinics and prisons.
What should be the focus of hepatitis C elimination programs in Australia?
Conclusion: Hepatitis C elimination programs in Australia should focus on increasing testing rates and linkage with care to maintain adequate levels of treatment.
How many treatments were prescribed by specialists in 2018?
During 1 January 2016 – 30 June 2018, 35 434 of 67 393 treatments for which the prescriber type was identified (53%) were prescribed by specialists. The proportion of treatments prescribed by non‐specialists has increased since 2016: from 13 117 of 34 130 treatments (38%) in 2016 to 12 763 of 23 635 (54%) in 2017, and 6079 of 9628 treatments (63%) in the first half of 2018 ( Box 3 ). This change in proportion was largely explained by declining numbers of treatments by specialists, as the numbers of treatments by non‐specialists were relatively stable.
How many HCV RNA tests are conducted annually?
During 1 January 2013 – 31 December 2015, 52 156 diagnostic HCV RNA tests were conducted (17 358 per year). During 1 January 2016 – 30 June 2018, 59 548 diagnostic HCV RNA tests were conducted (23 819 per year), including 32 519 tests (55%) for people in the 45–54 and 55–64‐year age groups ( Box 2 ). Increases in overall diagnostic testing before and after the introduction of DAAs were much less pronounced than increases in treatment uptake: the annual number of treatments during 2016–18 was almost triple that of 2013–2015, while the annual number of diagnostic RNA tests increased by 37%.
What is the purpose of HCV antibody test?
The presence of serum antibodies to the hepatitis C virus (HCV) indicates exposure to the virus; testing for HCV RNA (by polymerase chain reaction assay) is required to distinguish between an active infection and an earlier, resolved exposure. 10 The costs of HCV antibody and RNA testing are borne in Australia by the government through the Medicare Benefits Scheme. As the Medical Benefits Schedule (MBS) item number for HCV antibody tests is shared with a number of other common blood tests, they cannot be specifically identified in MBS data. The MBS lists three categories for HCV RNA testing: qualitative testing to confirm active hepatitis C infection; quantitative testing in preparation for treatment; and qualitative testing to confirm treatment success. Our analysis focused on diagnostic RNA testing; quarterly time series data on the number of diagnostic RNA tests, by age category and sex, were obtained for the period 1 January 2013 to 30 June 2018 from Medicare item reports ( http://medicarestatistics.humanservices.gov.au/statistics/mbs_item.jsp) ( Supporting Information, part A).
How can Australia meet the WHO 2030 elimination targets?
Modelling has indicated that Australia can meet the WHO 2030 elimination targets, provided treatment uptake can be sustained among people with advanced liver disease and people who inject drugs (the major group at risk of hepatitis C in Australia). 4 During the first 15 months of DAA treatment availability (March 2016 to June 2017), 44 382 treatment courses were initiated in Australia, 5 corresponding to 20% of the estimated 227 000 people with chronic hepatitis C in 2015. 6 While this level of treatment uptake exceeded the estimated 12% of patients per year required to reach the elimination targets (4725 treatments among the estimated 40 000 infected people who inject drugs), 4 early uptake reflected the availability of large numbers of patients waiting for DAAs to become accessible and easy‐to‐reach patients commencing DAA therapy. It is unclear whether high treatment rates can be sustained, and in some other countries they have declined. 7, 8 The level of reduction that would put achievement of the elimination targets in Australia at risk is unclear.
Is hepatitis C testing rate sufficient?
The known: Despite high initial uptake of hepatitis C treatment in Australia, it is uncertain whether the hepatitis C testing rate is sufficient to sustain the treatment up take necessary for achieving the WHO hepatitis C elimination targets by 2030.