
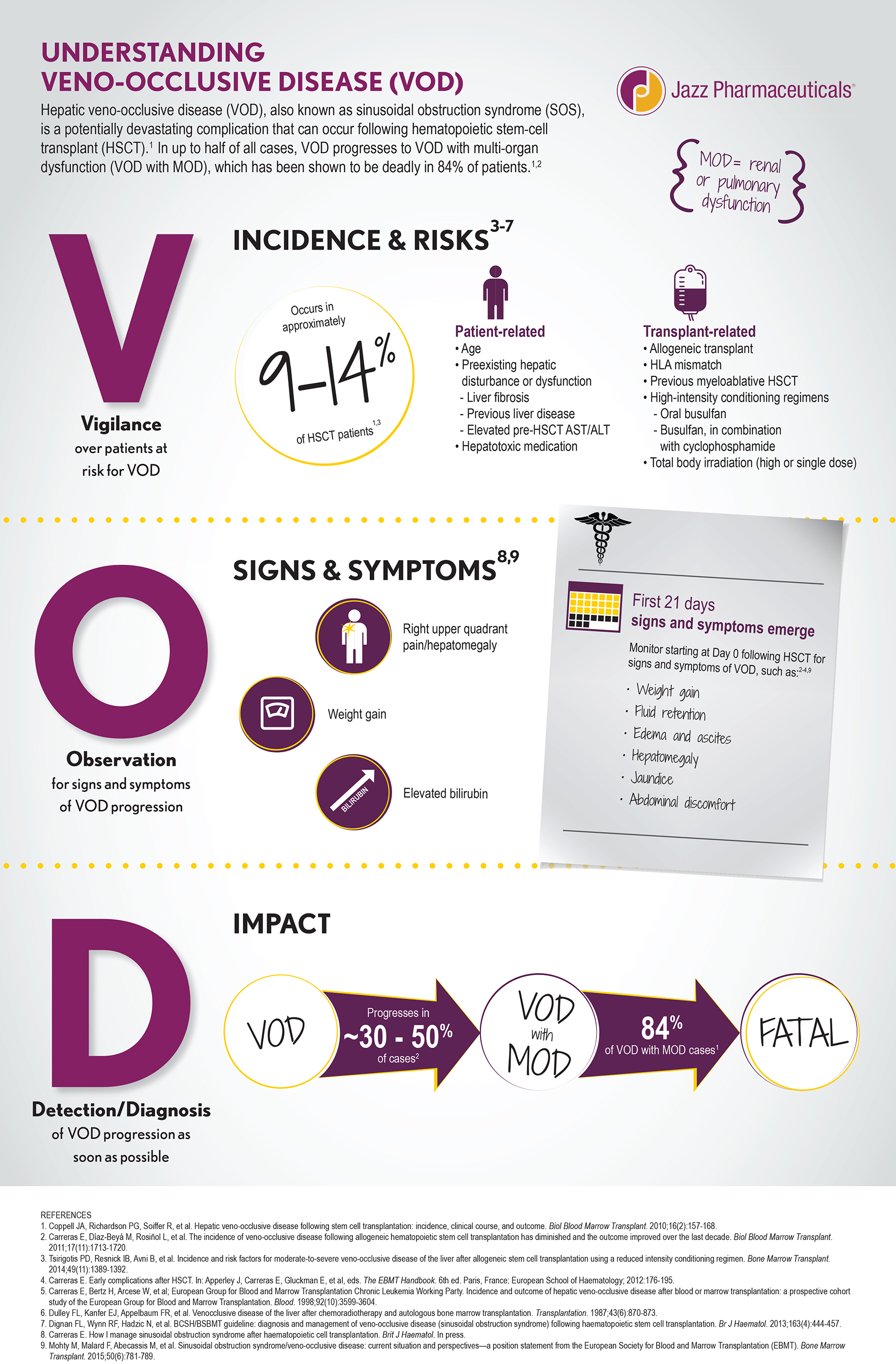
The U.S. Food and Drug Administration today approved Defitelio (defibrotide sodium) to treat adults and children who develop hepatic veno-occlusive disease (VOD) with additional kidney or lung abnormalities after they receive a stem cell transplant from blood or bone marrow called hematopoietic stem cell transplantation (HSCT).
Is Regenexx FDA approved?
The FDA does not “approve” or “not approve” medical procedures (like gall bladder surgery for example). Regenexx is considered a medical procedure. We are in compliance with all procedure codes; the FDA does not have any issues with the procedures we offer in the U.S.
What diseases can be treated with stem cell therapy?
- Multiple Sclerosis. Multiple sclerosis is a chronic disease where inflammation is caused by the failure of the immune system, which damages cells of the brain or spinal cord.
- Cardiovascular Diseases. ...
- Diabetes and Diabetic Ulcer. ...
- Autism. ...
- Liver Diseases. ...
- Crohn’s Disease. ...
- Fibromyalgia. ...
- Parkinson’s Disease. ...
- Injuries and Musculoskeletal Pain. ...
- COVID-19. ...
Should the FDA have approved eteplirsen?
The road to approval for eteplirsen has been rocky. In late April, as reported by Medscape Medical News, the FDA's Peripheral and Central Nervous System Drugs Advisory Committee concluded that studies of eteplirsen failed to provide persuasive evidence that the drug is effective in DMD. But the vote was close.
Is TMS therapy FDA approved?
The U.S. Food and Drug Administration (FDA) has approved the use of transcranial magnetic stimulation (TMS) as a new treatment option for obsessive-compulsive disorder (OCD). Following successful use in treating depression, the FDA officially expanded the therapy’s approval for OCD patients in August.
See more
When Will stem cells be FDA approved?
List of FDA approved stem cell therapies in 2022. The formal FDA list of approved drugs made from stem cells is called, appropriately enough, “Approved Cellular and Gene Therapy Products.” The current list is up to date as of June 1, 2022.
What is stem cells approved to treat?
People who might benefit from stem cell therapies include those with spinal cord injuries, type 1 diabetes, Parkinson's disease, amyotrophic lateral sclerosis, Alzheimer's disease, heart disease, stroke, burns, cancer and osteoarthritis.
Has stem cell therapy been approved?
Currently, the only stem cell treatments approved by the Food and Drug Administration (FDA) are products that treat certain cancers and disorders of the blood and immune system.
Are bone marrow stem cells FDA approved?
About FDA-approved Products Derived from Stem Cells These FDA-approved stem cell products are listed on the FDA website. Bone marrow also is used for these treatments but is generally not regulated by the FDA for this use.
What are the 4 types of stem cell therapy?
Stem cellsEmbryonic stem cells.Tissue-specific stem cells.Mesenchymal stem cells.Induced pluripotent stem cells.
How many cell therapies are FDA approved?
Today, more than 20 cell & gene products have been approved.
What is the most successful stem cell therapy?
The most successful stem cell therapy—bone marrow transplant—has been around for more than 40 years. Johns Hopkins researchers played an integral role in establishing the methods for how bone marrow transplants are done, which you can read about in Human Stem Cells at Johns Hopkins: A Forty Year History.
Which country is most advanced in stem cell therapy?
List of countries by stem cell research trialsRankCountry/TerritoryNumber of clinical trials1United States1362Iran653South Korea404Australia1810 more rows
Has the FDA-approved stem cell therapy for knees?
Currently, no stem cell treatments for arthritis are approved by the FDA. However, clinical trials are under way by some of the leading research hospitals and institutions in the United States.
Is PRP approved by the FDA?
Summary. While PRP is not 'FDA-approved', it can be legally offered in the clinic 'off-label' in the USA for a myriad of musculoskeletal indications.
Why is stem cell treatment not allowed in the US?
Stem cell research is legal in the United States, however, there are restrictions on its funding and use. Currently, the only stem cells now used to treat disease are from blood cell-forming adult stem cells found in bone marrow.
Is regenerative medicine FDA approved?
This web posting reemphasizes the warning to consumers in FDA's July 2020 Consumer Alert: Regenerative medicine therapies have not been approved for the treatment or prevention of COVID-19, acute respiratory distress syndrome (ARDS), or any other complication related to COVID-19.
What is the FDA's response to stem cell products?
When stem cell products are used in unapproved ways— or when they are processed in ways that are more than minimally manipulated, which relates to the nature and degree of processing—the FDA may take (and has already taken) a variety of administrative and judicial actions, including criminal enforcement, depending on the violations involved.
What is the FDA approved product?
About FDA-approved Products Derived from Stem Cells. The only stem cell-based products that are FDA-approved for use in the United States consist of blood-forming stem cells (hematopoietic progenitor cells) derived from cord blood. These products are approved for limited use in patients with disorders that affect the body system ...
What are stem cells?
Sometimes called the body’s “master cells,” stem cells are the cells that develop into blood, brain, bones, and all of the body’s organs. They have the potential to repair, restore, replace, and regenerate cells, and could possibly be used to treat many medical conditions and diseases. But the U.S. Food and Drug Administration is concerned ...
Where do stem cells come from?
The FDA has the authority to regulate stem cell products in the United States. Today, doctors routinely use stem cells that come from bone marrow or blood in transplant procedures to treat patients with cancer and disorders of the blood and immune system. Electron micrograph of stem cells, color-enhanced for visual clarity.
Do investigational products have to go through a FDA review?
With limited exceptions, investigational products must also go through a thorough FDA review process as investigators prepare to determine the safety and effectiveness of products in well-controlled human studies, called clinical trials. The FDA has reviewed many stem cell products for use in these studies.
Is stem cell treatment illegal?
Food and Drug Administration is concerned that some patients seeking cures and remedies are vulnerable to stem cell treatments that are illegal and potentially harmful. And the FDA is increasing its oversight and enforcement to protect people from dishonest and unscrupulous stem cell clinics, while continuing to encourage innovation so ...
Can stem cells be unsafe?
Please try again later. Researchers hope stem cells will one day be effective in the treatment of many medical conditions and diseases. But unproven stem cell treatments can be unsafe—so get all of the facts if you’re considering any treatment.
Ready to sign-up for cord blood banking?
Reserve your Cord Blood Collection Kit in time to bring it with you when you go into labor. Give your newborn every advantage with cord blood banking!
Donate Cord Blood
By donating your newborn’s cord blood, you are joining a nationwide effort to create a genetically diverse inventory of stem cells for transplant to a child.
Can a physician use PRP off label?
Physicians are allowed to use PRP for off-label use if the following guidelines are met. Physicians have the responsibility to be: well informed about the product, to base its use on firm scientific rationale and on sound medical evidence, and. to maintain records of the product’s use and effects.
Does the FDA test products?
It’s important to note that the FDA does not develop or test products. Instead, FDA experts review the results of laboratory, animal and human clinical testing done by manufacturers. This is part of the reason the Food and Drug Administration is slow to approve drugs and therapies. Native Stem Cell Clinics has been practicing stem cell therapy ...
Is stem cell therapy a good treatment for arthritis?
Research and experience show us that stem cell therapy is a beneficial treatment for joint pain and arthritis. FDA approval is not a limiting factor for doctors. Physicians do have the authority to make decisions, such as prescribing drugs for “off-label” use, based on medical expertise and evidence. Dr.
Is PRP approved by the FDA?
Patients frequently ask if PRP treatments and stem cell therapy have been approved by the FDA, and we want our patients to be as informed as possible. The FDA — as well as major physician organizations such as the American Academy of Orthopaedic Surgeons and the American Medical Association — are taking a close look at these regenerative medicine ...
What is the FDA approved product for stem cell?
Currently, the only stem cell products that are FDA-approved for use in the United States consist of blood-forming stem cells (also known as hematopoietic progenitor cells) that are derived from umbilical cord blood.
Who regulates stem cell products?
The US Food and Drug Administration (FDA) has authority to regulate regenerative medicine products, including stem cell products and exosome products. There is a lot of misleading information on the internet about these products, including statements about the conditions they can be used to treat. FDA is concerned that many patients seeking cures ...
What is the phone number for regenerative medicine?
If you are considering a regenerative medicine product and have questions about how it is regulated (including whether FDA approval is required), whether it is FDA-approved, or what to consider before participating in a clinical trial, we urge you to call (800-835-4709) or email ([email protected]) for information.
Is FDA misled?
FDA is concerned that many patients seeking cures and remedies may be misled by information about products that are illegally marketed, have not been shown to be safe or effective, and, in some cases, may have significant safety issues that put patients at risk.
Is exosome approved for other uses?
These products are approved for use in patients with disorders that affect the production of blood (i.e., the “hematopoietic” system) but they are not approved for other uses. Exosome products are also regulated by FDA.
Does the FDA have information on regenerative medicine?
FDA has posted information for consumers and patients that discusses the potential risks, and provides advice for people considering the use of these products. Consumers should be cautious of any clinics, including regenerative medicine clinics, or health care providers, including physicians, chiropractors, or nurses, ...
What diseases does a stem cell treatment for?
The company, through its affiliated centers or clinics throughout the U.S., offers unapproved stem cell products to treat a variety of diseases and conditions, such as Lyme disease, diabetes, Parkinson’s disease, stroke, kidney failure and amyotrophic lateral sclerosis (ALS).
When will the FDA issue premarket approval?
The agency noted that it intends to exercise enforcement discretion for certain products until November 2020 with respect to the FDA’s investigational new drug application and premarket approval requirements when the use of the product does not raise reported safety concerns or potential significant safety concerns.
Why is the FDA on notice?
FDA puts company on notice for marketing unapproved stem cell products for treating serious conditions.
How to file a report for MedWatch?
To file a report, use the MedWatch Online Voluntary Reporting Form. The completed form can be submitted online or via fax to 1-800-FDA-0178. The FDA monitors these reports and takes appropriate action necessary to ensure the safety of medical products in the marketplace. ###.

Stem Cell Uses and FDA Regulation
- The FDA has the authority to regulate stem cell products in the United States. Today, doctors routinely use stem cells that come from bone marrow or blood in transplant procedures to treat patients with cancer and disorders of the blood and immune system. With limited exceptions, investigational products must also go through a thorough FDA review p...
Safety Concerns For Unproven Stem Cell Treatments
- All medical treatments have benefits and risks. But unproven stem cell therapies can be particularly unsafe. For instance, attendees at a 2016 FDA public workshopdiscussed several cases of severe adverse events. One patient became blind due to an injection of stem cells into the eye. Another patient received a spinal cord injection that caused the growth of a spinal tumo…
FDA Actions on Unapproved Stem Cell Products
- When stem cell products are used in unapproved ways—or when they are processed in ways that are more than minimally manipulated, which relates to the nature and degree of processing—the FDA may take (and has already taken) a variety of administrative and judicial actions, including criminal enforcement, depending on the violations involved. In August 2017, the FDA announce…