Treatment
- Medication. High-dose corticosteroids may be used to ease swelling around the tumors and decrease the neurological signs and symptoms.
- Surgery. ...
- Radiation therapy. ...
- Rehabilitation after treatment. ...
- Supportive (palliative) care. ...
- Alternative medicine. ...
Full Answer
What to expect while receiving radiation therapy to the brain?
· Radiation Therapy to the Brain About Radiation Therapy to the Brain. Radiation therapy uses high-energy rays to treat cancer. It works by damaging the... Simulation. Before you begin your treatment, you’ll have a treatment planning procedure called a simulation. ... Your... After Your Simulation. ...
How long does it take to recover from radiation treatment?
Whole brain radiotherapy (WBRT) is the treatment of choice for the majority of class 1 patients. Most of the Class 2 patients are not suitable for WBRT. Class 3 patients are treated with supportive care only. The standard radiotherapy regime is either 20 Gy in …
What are the side effects of full brain radiation?
· Whole brain radiotherapy (WBRT) is a mainstay of treatment in patients with both identifiable brain metastases and prophylaxis for microscopic disease. The use of WBRT has …

How long does it take to recover from whole brain radiation?
You may develop fatigue after 2 to 3 weeks of treatment, and it can range from mild to severe. Fatigue may last 6 weeks to 12 months after your treatment ends. There are a lot of reasons why you may develop fatigue during treatment, including: The effects of radiation on your body.
What is the success rate of whole brain radiation?
The median WBRT-free survival was 8.5 months (range 0.8–107.3 months) with 30% ultimately requiring salvage WBRT. One hundred and four patients (34%) survived beyond 1 year without the need for salvage WBRT, while 56 patients (18%) either died or required WBRT within 3 months.
Does whole brain radiation improve survival?
Similarly, the survival benefit of WBRT to brain metastases is controversial. A randomized clinical trial QUARTZ showed that except for younger patients, WBRT had no significant effect on survival or quality of life in patients with brain metastases [18].
What is the purpose of whole brain radiation?
A type of external radiation therapy used to treat patients who have cancer in the brain. It is often used to treat patients whose cancer has spread to the brain, or who have more than one tumor or tumors that cannot be removed by surgery.
Is whole brain radiation painful?
If the cancer is in many areas, sometimes the whole brain is treated with radiation. The side effects of whole brain radiation therapy may not be noticeable until a few weeks after treatment begins. Radiation to the brain can cause these short-term side effects: Headaches.
What are the long term effects of whole brain radiation?
WBRT's side effects include hair loss, skin redness, dry mouth and fatigue. It is associated with significant interruptions in systemic therapy. In contrast, side effects associated with radiosurgery are minimal, and it's generally not associated with significant interruptions in chemotherapy, says Brown.
How many times can you have whole brain radiation?
Whole-brain radiation applies radiation to the entire brain in order to kill tumor cells. People undergoing whole-brain radiation usually require 10 to 15 treatments over two to three weeks. Side effects may include fatigue, nausea and hair loss.
What does whole brain radiation feel like?
Some short-term memory loss and difficulty thinking can occur if you are treated with whole-brain radiation therapy. Brain tissue swelling can develop during treatment. You may get a headache or feel pressure in your head if this occurs.
How long does it take for a brain tumor to shrink after radiation?
This can occur six months to a few years after treatment. However, there is less risk of necrosis today because of newer, targeted radiation therapies and the emergence of powerful imaging, brain mapping and information technologies.
What are the final stages of a brain tumour?
What Are the Symptoms of End-Stage Brain Cancer?Frequent headaches.Agitation and delirium.Agonal breathing (gasping breaths that occur when a person is struggling to breathe)Prolonged confusion.Hallucinations.Loss of appetite.Vision loss.Involuntary movements.More items...
Will radiation shrink a tumor?
Radiation therapy kills cancer cells or slows their growth by damaging their DNA. Radiation therapy (also called radiotherapy) is a cancer treatment that uses high doses of radiation to kill cancer cells and shrink tumors.
Can radiation get rid of a brain tumor?
Radiation therapy is used to shrink tumors and slow the growth of brain cancer. It's often used together with chemotherapy or surgery to give doctors the best chance of completely removing the tumor. It's also used for people who aren't able to undergo surgery.
How does radiation therapy work?
Radiation therapy uses high-energy rays to treat cancer. It works by damaging the cancer cells and making it hard for them to reproduce. Your body then is naturally able to get rid of these damaged cancer cells. Radiation therapy also affects normal cells.
How to quit smoking after radiation?
Telling your doctor or nurse if you’re in pain. Caring for yourself at home: Quitting smoking, if you smoke. If you want to quit, call our Tobacco Treatment Program at 212-610-0507. Following your radiation therapy team’s instructions to care of your skin.
How to help someone with cancer?
Join a support group. Meeting other people with cancer will give you a chance to talk about your feelings and listen to other people who have the same concerns. You’ll learn how others cope with their cancer and treatment. Your doctor, nurse, or social worker can tell you about the support groups you might be interested in.
Is cancer treatment stressful?
Cancer diagnosis and treatment can be very stressful and overwhelming. You might feel:
Can you pass radiation to someone else?
You might have concerns about how cancer and your treatment can affect your sexuality. You aren’t radioactive. You can’t pass radiation to anyone else, so it’s safe to be in close contact with other people.
Can you have radiation therapy with stereotactic radiosurgery?
You’ll have either external beam radiation therapy or stereotactic radiosurgery depending on your treatment plan. During external beam radiation, a treatment machine will aim beams of radiation directly to the tumor. The beam passes through your body and destroys cancer cells in its path.
Does radiation therapy affect normal cells?
Radiation therapy also affects normal cells. However, your normal cells are able to repair themselves in a way that cancer cells can’t. Radiation can be given to treat primary tumors in your brain or tumors that have spread to your brain from another part of your body (metastasized).
What is WBRT treatment?
WBRT is one of the most effective modalities for the treatment and prevention of brain metastases, although it can result in neurocognitive deficits . WBRT is associated with the development of delayed white matter abnormalities or leukoencephalopathy and has been correlated with cognitive dysfunction. The effects of WBRT have been studied in the setting of treatment for intracranial disease and prophylactic cranial irradiation. Prophylactic cranial irradiation is ideal for studying the effect of WBRT, as patients do not have baseline neurologic effects from metastatic or primary tumors in the brain.
How long does it take to recover from brain metastases?
Based on the results of multiple RTOG trials conducted in the 1970s and 1980s, it is established that patient survival is prolonged from 1 to 2 months with supportive care alone to 3-6 months with a standard regime of 30 Gy delivered in 10 fractions. 62,75,76
Is WBRT a palliative treatment for NSCLC?
WBRT is the mainstay of palliative treatment for NSCLC with brain metastasis. Preclinical experiments have shown that NSCLC cell lines that carry EGFR mutations are much more sensitive to radiation, and their clonogenic survival in response to radiation was reduced 500–1000-fold compared to the wild-type NSCLC cell lines ( Das et al., 2006 ). In supporting this concept, Gow et al. (2008) reported that patients with EGFR mutations had a higher response rate (54%) to WBRT compared with those with the wild type (24%) from a retrospective analysis from their Taiwanese database. In addition, TKIs are radiation sensitizers in in vitro studies ( Chinnaiyan et al., 2005 ). Therefore, there is sound rationale to combine EGFR-TKI with WBRT for NSCLC patients with brain metastases. The hypothesis was that EGFR inhibition can be combined with WBRT to enhance the therapeutic ratio by selectively targeting the cancer cells within the brain, thereby extending the survival without increasing the neurotoxicity associated with WBRT ( Welsh et al., 2013 ).
Is WBRT a first line treatment for brain metastasis?
While whole brain radiation therapy (WBRT), due to its toxicity and cognitive complications, has not been recommended as the first-line therapy of brain metastasis ( Richards et al., 2007 ), WBRT is an accepted treatment modality used in poor prognostic patients with active extracranial tumor and/or multiple intracranial metastatic lesions ( Song et al., 2014 ). To be eligible for WBRT, patients should have a Karnofsky performance score (KPS) of greater than 70 and be capable of caring for themselves independently ( Yoshida, 2007 ). Increased survival, local control of the metastasis, improvement in neurological function, and control of undiagnosed micrometastases are observed after WBRT ( Andrews et al., 2004; Kondziolka et al., 2005; Roberge et al., 2009 ).
How long does WBRT last?
Studies have demonstrated that WBRT resulted in a median survival of only 2–6 months in patients with melanoma brain metastases but did improve neurologic function and was superior to the use of steroids only ( Stridsklev et al., 1984 ). Melanoma has often been viewed as radioresistant and retrospective analysis of patients treated with WBRT have shown that brain lesions contributed to death nearly in 95% cases ( Sampson et al., 1998 ). Melanoma brain metastases appear to have worse response and local control to WBRT than brain metastases of other histologies ( Nieder et al., 1997 ). Whole-brain radiation remains the standard of care in the setting of nonoperative large-volume tumors (i.e., >2 cm), numerous brain metastases, and for patients who have failed initial SRS or surgical resection ( Ramakrishna and Margolin, 2013 ).
What is WBRT in medical terms?
Multiple brain metastases. Whole brain radiotherapy (WBRT) is the treatment of choice for the majority of class 1 patients. Most of the Class 2 patients are not suitable for WBRT. Class 3 patients are treated with supportive care only.
Does WBRT help with metastases?
WBRT has, until recently, been the treatment of choice in patients with metastases. However, the QUARTZ trial has highlighted that poor performing patients may do better with supportive corticosteroids instead. The trial randomized patients to WBRT plus steroids or supportive care with steroids alone. Median survival was 8.5 weeks in both groups, with no difference in quality-of-life endpoints. Although the trial has been criticized for having few patients of good performance i.e., patients of GPA 3 and above, its intentions were to omit WBRT in the worst predicted population, i.e., patients with a GPA of 2 and less. Subgroup analysis within the trial demonstrated no difference in survival between high and low scoring GPA assessments ( Mulvenna et al., 2016 ). It must not be forgotten that the addition of WBRT improves distant in brain control by approximately 50% and increases local control by 15%. No change in overall survival is seen due to the salvage option of SRS ( Aoyama et al., 2006; Chang et al., 2009; Kocher et al., 2011; Brown et al., 2016 ).
How to deliver radiation to brain metastases?
Another strategy to deliver higher doses of radiation to brain metastases is by using standard WBRT in conjunction with simultaneous in-field boost (SIB) delivered through helical tomotherapy. Rodrigues et al. developed a dosimetric feasibility study that demonstrated the possibility of the administration of an SIB dose of 60 Gy in 10 fractions that is biologically equivalent to a single SRS dose of 18 Gy but superior in terms of normal tissue tolerance; a Phase I trial assessing toxicity has already been accomplished based upon this feasibility study, which demonstrated minimal toxicity.[69] A Phase II trial conducted by the same research group is currently underway, with the goal of comparing the efficacy of helical tomotherapy SIB versus traditional SRS as an adjunct to WBRT.[68]
What is WBRT treatment?
Whole brain radiotherapy (WBRT) is a mainstay of treatment in patients with both identifiable brain metastases and prophylaxis for microscopic disease. The use of WBRT has decreased somewhat in recent years due to both advances in radiation technology, allowing for a more localized delivery of radiation, and growing concerns regarding the late toxicity profile associated with WBRT. This has prompted the development of several recent and ongoing prospective studies designed to provide Level I evidence to guide optimal treatment approaches for patients with intracranial metastases. In addition to defining the role of WBRT in patients with brain metastases, identifying methods to improve WBRT is an active area of investigation, and can be classified into two general categories: Those designed to decrease the morbidity of WBRT, primarily by reducing late toxicity, and those designed to improve the efficacy of WBRT. Both of these areas of research show diversity and promise, and it seems feasible that in the near future, the efficacy/toxicity ratio may be improved, allowing for a more diverse clinical application of WBRT.
Where are NSCs located in the brain?
Specifically, these NSCs were identified to be located within a small area of the hippocampus known as the subgranular zone as well as in the subventricular zones. [49] It is proposed that these NSCs are capable of repairing damage to both white and gray matter in the brain, and when destroyed by radiation may prevent neural regeneration and cause subsequent neurocognitive toxicity.[26] NSC sparing has also been shown to be possible by Marsh et al. through feasibility studies, and trials of NSC sparing in patients with cerebral metastases have been proposed.[51]
What areas of the brain are sparing?
One of the first of these areas to be characterized as a potential candidate for sparing is the hippocampus. As memory loss (particularly the loss of the ability to consolidate new memories) is one of the more common and subjectively detrimental late adverse effects of WBRT, the hippocampus seemed a natural target to avoid. Additionally, metastatic lesions to the hippocampus are extremely rare, and in approximately 86% of patients with cerebral metastases, there exists at least a 15 mm margin between the closest metastasis and the hippocampus, which would allow for effective sparing with modern WBRT without compromising therapeutic efficacy.[32] Sparing of the hippocampal region seems to be particularly important in the pediatric population, as it has been conclusively demonstrated that decrease in IQ and ability to form new memories correlated with radiation exposure to the temporal regions of the brain containing the hippocampus.[54,59] Hippocampal sparing has been shown to be feasible by multiple radiation treatment planning studies, and is likely to receive increasing attention in the future for PCI,[52,67,88] and is already currently being investigated by the RTOG in an ongoing clinical trial.[71] In addition to the hippocampus itself, other contiguous structures of the limbic system have been proposed as candidates for avoidance during administration of WBRT due to their involvement in memory consolidation, emotional processing, and fine motor coordination—all processes that seem to be negatively affected by irradiation of the whole brain. Similar to the hippocampus, the limbic system as a whole seems to only rarely harbor metastatic disease and sparing is dosimetrically feasible.[50,52]
What is WBRT used for?
In addition to treating patients with known brain metastases, WBRT is also used to treat cancer patients with a high risk of developing brain metastases, termed PCI. The possible utility of PCI for prevention of small cell lung cancer (SCLC)-associated intracranial metastases is one that has been recognized and studied since the 1970s, but individual trials conducted in the mid-1990s were unable to unanimously prove PCI's efficacy in decreasing mortality.[6] In 1999, Auperin et al. published the first meta-analysis that was able to demonstrate a survival benefit of PCI.[6] This study analyzed the results of seven randomized clinical trials (RCTs) in which PCI was offered to SCLC patients who had a complete response to therapy, and found that there was a decrease in the incidence of brain metastasis at 3 years in patients who received PCI versus those who did not (33% versus 59%, respectively), along with a decrease in mortality (79.3% versus 84.7%, respectively).[6] Recent trials have attempted to extend these findings in patients with an incomplete response to chemotherapy. A recent study completed by the EORTC demonstrated both a decreased risk of developing brain metastasis and improvement in overall survival at one year in patients receiving PCI.[76] PCI has also been studied in patients with nonsmall cell lung cancer (NSCLC). A trial conducted by the RTOG demonstrated a decrease in the incidence of brain metastasis in the PCI arm (8% with PCI versus 18% in the observation arm), however, there was no statistically significant difference in overall survival.[34]
What is the treatment for multiple cerebral metastases?
In patients with multiple cerebral metastases, WBRT is generally the treatment of choice, as it addresses both macroscopic and microscopic disease. Studies have shown an improvement in symptoms in 64-83% of patients after treatment with WBRT alone,[10,40,77] and have also demonstrated an increase in median OS from 1 month with no treatment to 3-7 months following WBRT.[30] The most common dose/fractionation schedule used is 30 Gy delivered in 10 fractions over the course of 2 weeks.[10,77] There have been a number of trials that have utilized alternative dose-fractionation schedules, including protocols with both lower and higher doses of overall radiation compared with the control regimens. Most of these studies have failed to show a statistically significant difference, although a trial performed by Davey et al. was able to show an increase in time before intracranial relapse of 32 weeks in the experimental dose arm (40 Gy/20 BID fractions) versus 14 weeks in the control arm (20 Gy/5 fractions).[17] Toxicity associated with the alternative dose-fractionation schedules reported in the literature is not significantly different than standard schedules, with the exception of high dose single fraction therapy at doses of 10 Gy, which result in greater toxicity.[38] Overall, sufficient evidence is not currently available to suggest superior efficacy or reduced toxicity of alternative dosing schedules over the accepted and currently used standard WBRT protocols.[82] Therefore, prospective studies with carefully designed neurocognitive endpoints are still needed to more clearly determine if altered fractionation schedules may be beneficial, particularly in the context of brain metastasis patients with the potential for long-term survival.
Is WBRT a local toxicity?
Radiation necrosis is perhaps the most extreme local toxicity to occur with WBRT. As the name implies, areas of the brain affected by radiation necrosis represent necrotic, nonreparable tissue. Pathologically, these areas are characterized by fibrinoid necrosis of small arteries and arterioles, which is hypothesized to be the result of extensive damage to the vascular endothelium.[11,86] Although more common in patients being treated with higher doses of radiation to limited areas of the brain, such as in treatment with SRS, WBRT has also been implicated as a cause of radiation necrosis. However, this late effect is relatively uncommon, as the general threshold to develop radiation-induced necrosis typically exceeds doses used in traditional WBRT dose-fractionation schedules.[22,48]
Types of Radiation Therapy for Brain Tumors
There are 2 main types of therapy. You may get both types. They include:
External beam radiation therapy (EBRT)
There are several types of EBRT. The goal is to target the tumor and limit damage to nearby healthy brain cells. To limit the harm, your healthcare provider may use special types of EBRT such as:
Brachytherapy
For this treatment, the radiation is placed very close to or inside the tumor. This is done during surgery. The radiation the implants give off travels a very short distance. This helps limit the effect on nearby healthy tissue.
Brain Radiation Side Effects
Generally, side effects from radiation treatment are grouped into two categories:
Radiation necrosis
Sometimes dead brain tissue forms at the site of the radiation. This is called radiation necrosis. The mass of dead brain tissue comes from both cancer cells and healthy cells. Radiation necrosis can take anywhere from months to years to develop.
Risk of future cancer
Radiation can damage the DNA in healthy cells. As a result, you have a small risk of a second brain cancer after brain radiation. This second cancer usually occurs many years later. Talk to your radiation oncologist about the risks and benefits of radiation therapy.
The Radiation Team
Treatment planning for radiation therapy includes mapping to pinpoint the exact location of the brain tumor using X-rays or other images.
What is the treatment for cancer of the brain?
Cancer that spreads to the brain is usually treated with radiosurgery - highly focused radiation with a tool such as the Gamma Knife, followed by less intense radiation to the whole brain. The latter treatment can cause hair loss, dry mouth, fatigue and thinking problems.
Does radiation therapy help with brain cancer?
Contrary to conventional wisdom, radiation therapy to the whole brain did not improve survival, and it harmed memory, speech and thinking skills, doctors found.
Does radiation help cancer?
Radiation helped control the cancer, "but at the cost of cognitive decline.". For patients, the study is not necessarily the bad news it may seem. It shows that in this case, quality of life is better with less treatment, and many people can be spared the expense and side effects of futile care.
What is WBRT in medical terms?
Whole Brain Radiation Therapy (WBRT) —the conventional radiation therapy method for brain metastases, whole brain radiotherapy, has been in use since 1954 [1,2] even though there is a risk of short- and long-term side effects [3,4]. WBRT is just what it sounds like—giving radiation to the entire brain, even to healthy tissue.
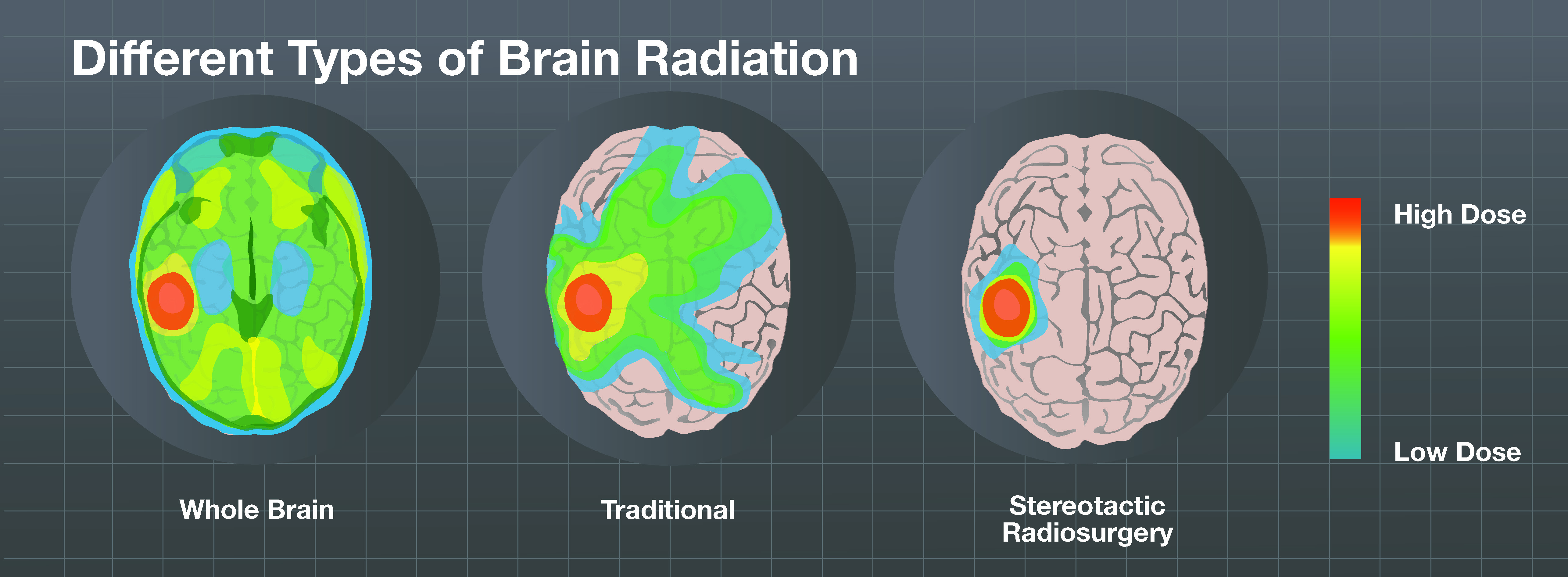
What is stereotactic radiosurgery?
Stereotactic radiosurgery is an advanced and targeted radiation delivery technique. Brain metastases are typically small lesions and can often be effectively controlled with the precise administration of a large dose of radiation directly to the tumor.
What is LINAC therapy?
For many cancers, LINAC therapy is one of the most precise and advanced forms of radiation treatment available. Cobalt-60 Therapy —radiation for this treatment comes from a gamma-emitting radioactive isotope of cobalt, which is used to treat tumors. Unlike LINAC therapy, patients are put under local anesthesia for treatment delivery.
Can radiation therapy stop brain tumors?
Sophisticated treatment machines deliver high doses of radiation to the tumor target (s) to stop or slow growth. Radiation can be used alone or in addition to surgery or chemotherapy. If your cancer care team recommends radiation therapy to treat your brain metastases, you will want to explore the different types available and understand ...
Does radiation therapy help with brain metastases?
As mentioned above, the conventional radiation therapy method for brain metastases is whole brain radiotherapy based on the common theory that, because primary cancer cells break off and travel around the body through the bloodstream and lymph system, the entire brain is “seeded” with metastases, even if only a single tumor is found.
How long does it take for radiation to show up in the brain?
Radiation to the brain can also have side effects that show up later – usually from 6 months to many years after treatment ends. These delayed effects can include serious problems such as memory loss, stroke-like symptoms, and poor brain function.
How to reduce side effects of radiation?
One way to reduce side effects is by using radioprotective drugs, but these are only used for certain types of radiation given to certain parts of the body. These drugs are given before radiation treatment to protect certain normal tissues in the treatment area. The one most commonly used today is amifostine.
Can radiation cause rib fractures?
Rib fractures: In rare cases, radiation therapy may weaken the ribs, which could lead to a fracture. Be sure you understand what to look for and tell your cancer care team if you notice any of these side effects. Heart complications: Radiation to the breast can also affect the heart.
How long does it take for side effects to show up after radiation?
Some side effects might show up quickly, but others might not show up until 1 to 2 years after treatment. Talk with your radiation oncologist about what to watch for and when to call your doctor. If the cancer is in many areas, sometimes the whole brain is treated with radiation.
Can you get radiation therapy for brain tumors?
If you’re getting radiation therapy to the brain. People with brain tumors often get stereota ctic radiosurgery (radiation given in one large dose) if the cancer is in only one or a few sites in the brain. Side effects depend on where the radiation is aimed.
Can radiation therapy cause low blood count?
Rarely, radiation therapy can cause changes in your blood count levels. These blood cells help your body fight infection and prevent bleeding. If your blood tests show low blood counts, your treatment might be stopped for a week or so to allow your blood counts to return to normal. This side effect is more likely if you’re also getting chemotherapy.
What is the most common drug used for radiation therapy?
The one most commonly used today is amifostine. This drug may be used in people with head and neck cancer to reduce the mouth problems caused by radiation therapy. Not all doctors agree on how these drugs should be used in radiation therapy. These drugs have their own side effects, too, so be sure you understand what to look for.