What are the treatment options for hepatitis C virus (HCV) genotype 2?
For initial treatment of HCV genotype 2 in adults with compensated cirrhosis, the recommended regimens are sofosbuvir-velpatasvir for 12 weeks or glecaprevir-pibrentasvir for 8 weeks. If the individual has HCV-HIV coinfection, and compensated cirrhosis, the glecaprevir-pibrentasvir treatment should be extended to 12 weeks.
What is the AASLD-IDSA HCV guidance for initial treatment of chronic HCV genotype 2?
The following is a summary of the AASLD-IDSA HCV Guidance for initial treatment of persons with chronic HCV genotype 2 infection. [ 20, 21] For individuals with cirrhosis, the AASLD-IDSA HCV Guidance defines compensated cirrhosis as Child-Turcotte-Pugh class A and decompensated cirrhosis as Child-Turcotte-Pugh class B or C.
What factors influence the optimal regimen for retreatment of HCV genotype 2?
For retreatment of adults with HCV genotype 2, four major factors influence the optimal regimen for retreatment, including (1) the prior regimen the patient failed, including whether there was prior exposure to an NS5A inhibitor, (2) the presence or absence of cirrhosis, (3) cost or insurance considerations.
What is the best treatment for genotype 2 cirrhosis of the liver?
In particular, very limited data exist with retreatment of genotype 2 patients with cirrhosis. Recent trial data suggest either of the pangenotypic combinations of sofosbuvir-velpatasvir or glecaprevir-pibrentasvir are highly effective in treatment-experienced patients with HCV genotype 2 infection.
What does HCV genotype 2 mean?
Hepatitis C genotype 2 is often curable. But chronic infection can lead to serious complications. Most people with hepatitis C experience no symptoms or only mild symptoms, even when the liver is becoming damaged. The first six months after infection is defined as acute hepatitis C infection.
Which HCV genotype is easiest to treat?
In the United States, hepatitis C genotype 3 is less commonly contracted than genotype 1, but genotype 3 is also harder to treat....Genotype 3 has been found to respond better to newer drug combinations, including:glecaprevir-pibrentasvir (Mavyret)sofosbuvir-velpatasvir (Epclusa)daclatasvir-sofosbuvir (Sovaldi)
Which HCV genotype is hardest to treat?
Summary PointsIn the DAA era, HCV genotype 3 has emerged as the most difficult HCV genotype to treat.For treatment-naïve adults without cirrhosis, two regimens are recommended with equal evidence rating: (1) glecaprevir-pibrentasvir for 8 weeks, or (2) sofosbuvir-velpatasvir for 12 weeks.More items...•
Can all genotypes of hep C be cured?
Once-daily combination pills that can treat all genotypes of hepatitis C infection are curing almost everyone who completes a course of treatment, and drop-out rates during treatment are low, large 'real-world' cohort studies reported this week at The International Liver Congress in Vienna.
Can HCV genotype change?
Six major genotypes of the hepatitis C virus (HCV) have been described; it is assumed to be uncommon for genotypes to change in chronically infected individuals.
How many HCV genotypes are there?
HCV genomic analysis by means of an arduous gene sequencing of many viruses has led to the division of HCV into six genotypes based on homology. Numerous subtypes have also been identified. Arabic numerals denote the genotype, and lower-case letters denote the subtypes for lesser homology within each genotype.
Which genotype is most common?
Hepatology. 2014;doi:10.1002/hep. 27259. Hepatitis C virus genotype 1 is the most prevalent genotype and accounts for 83.4 million infections, or 46.2% of all cases, worldwide, according to a study published in Hepatology.
Why is HCV genotype important?
Knowing your HCV genotype is important information that can help patients and doctors find the most effective treatment. All HCV genotypes cause the same amount of liver damage.
How is Hep C genotype determined?
HCV genotyping methods The genotype of HCV for diagnosis is mostly determined by sequencing of genomic nucleotide sequence or by kit-based assays which employ complementary probes to report genotype present in a specimen.
Does hep C go away after treatment?
Hepatitis C infection is cured if the virus is undetectable 12 weeks after the completion of a course of direct-acting antiviral treatment. This is known as a sustained virologic response (SVR).
How long is hep C treatment?
How long is the treatment? Treatment is usually 8-12 weeks long but can be as much as 16 weeks long in certain situations. Some patients with more damage to their liver may require 24 weeks of treatment, but this is uncommon. The duration depends on the medication, and specific HCV factors in particular patients.
Can hep C heal itself?
Like the human papillomavirus (HPV), early acute hepatitis C can clear on its own without treatment; this happens about 25% of the time. However, it's more likely that the virus will remain in your body longer than six months, at which point it's considered to be chronic hepatitis C infection.
What is the SVR rate for HCV genotype 2?
Historically, in the interferon era, treatment of persons with HCV genotype 2 infection achieved higher sustained virologic response (SVR) rates than those with HCV genotype 1 infection, even with a shorter duration of therapy and lower doses of ribavirin. Prior to the availability of DAAs, the standard of care for treatment-naïve patients with HCV genotype 2 consisted of a 24-week course of peginterferon plus fixed-dose ribavirin, with SVR rates of 75 to 85%. [ 6, 7, 8, 9] In 2013, the combination of sofosbuvir with peginterferon and ribavirin showed greater than 90% SVR12 rates in HCV genotype 2 infection. [ 10] Later that year, the FDA approved a 12-week course with the all-oral regimen of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection based on data from several studies showing SVR rates of approximately 92 to 97% with this regimen. [ 11, 12, 13] In 2015, daclatasvir plus sofosbuvir was FDA-approved as the first interferon- and ribavirin-free combination for HCV genotype 2 infection and this 12-week combination produced SVR rates of greater than 95%. [ 14, 15] Subsequently, SVR rates of 99% have been reported with sofosbuvir-velpatasvir or glecaprevir-pibrentasvir for initial treatment of individuals with HCV genotype 2. [ 16, 17, 18, 19]
What are the factors that influence the choice of treatment for genotype 2?
For patients chronically infected with genotype 2 HCV, two key factors influence the choice and duration of therapy: cirrhosis status and prior treatment experience. In addition, the cost of the regimen, insurance coverage, concurrent medications, and patient and provider preference can play a major role in the regimen choice. The following treatment recommendations are based on the AASLD-IDSA HCV Guidance for initial treatment of adults with HCV genotype 2 and for retreatment of adults in whom prior therapy failed, including those with HCV genotype 2. [ 4, 5]
What is the AASLD-IDSA HCV guidance?
The following is a summary of the AASLD-IDSA HCV Guidance for adults with HCV genotype 2 infection who failed prior DAA therapy , including those without cirrhosis and those with compensated cirrhosis. [ 24, 25, 26] For these purposes, compensated cirrhosis is defined as Child-Turcotte-Pugh class A and decompensated cirrhosis as Child-Turcotte-Pugh class B or class C. The AASLD-IDSA HCV Guidance for retreatment is no longer genotype specific, but instead emphasizes a pangenotypic approach to retreatment based on the prior treatment regimen. In addition, the AASLD-IDSA HCV Guidance no longer includes recommendations for the retreatment of persons who experienced prior treatment failure with interferon-based therapy, including interferon plus first-generation protease inhibitors ( telaprevir , boceprevir ); these individuals have robust cure rates with modern DAA regimens similar to that observed with treatment-naïve persons. The recommended retreatment regimens are based on prior regimen failure and listed by evidence level; when the evidence level is considered equivalent, the regimens are listed alphabetically.
What is genotype 2?
In the United States, genotype 2 accounts for approximately 13 to 15% of all hepatitis C virus (HCV) infections. [ 1] In the era before direct-acting antiviral agents (DAAs), sustained virologic response rates at 12 weeks post-treatment (SVR12) were relatively higher in persons with genotype 2 HCV than those with genotype 1, 3, or 4 HCV. Thus, data regarding retreatment of individuals with genotype 2 in whom prior therapy failed are limited. The following discussion regarding initial treatment and retreatment of persons with genotype 2 chronic HCV assumes the individual and their clinician have already made the decision to proceed with hepatitis C therapy. This topic review does not address the treatment of HCV genotype 2 in persons with decompensated cirrhosis, severe renal impairment (or end-stage renal disease), or post-liver transplantation.
Is genotype 2 retreatment less clinical experience than genotype 1?
Accordingly, less clinical experience exists with retreatment of patients with genotype 2 than with genotype 1 infection. In particular, very limited data exist with retreatment of genotype 2 patients with cirrhosis.
The Goal Of Hepatitis C Therapy
Hepatitis C virus was discovered by Choo et al. in the United States of America in 1989, and it has become clear that at least 90% of patients who were diagnosed as non-A, non-B hepatitis and at least half of all patients who were diagnosed as alcoholic liver diseases have liver damages caused by HCV.
Factors To Consider Prior To Choosing Retreatment Regimen
For retreatment of adults with HCV genotype 2, four major factors influence the optimal regimen for retreatment, including the prior regimen the patient failed, including whether there was prior exposure to an NS5A inhibitor, the presence or absence of cirrhosis, cost or insurance considerations.
Outcome Of Patients With Genotype 2 Or 3
Patients with genotype 2 or 3 showed an initial virological response rate of 100% under daily dose IFN-2a treatment. During treatment, however, three genotype 3 patients were excluded from the study due to incompliance. Thus, at that time the drop outs were virological responders.
Epidemiologyglobal Comparison And Resource Factors
When the epidemiology of HCV infection globally is being discussed, it is imperative to discuss northwest and eastsouth differences as well.
Hyperlipidemia Diabetes Mellitus Or Ir
Lipid metabolism is intimately involved in the molecular mechanisms of the HCV infectious cycle. HCV replication influences and depends upon cholesterol uptake and efflux through different lipoprotein receptors during its entry into the hosts cells .
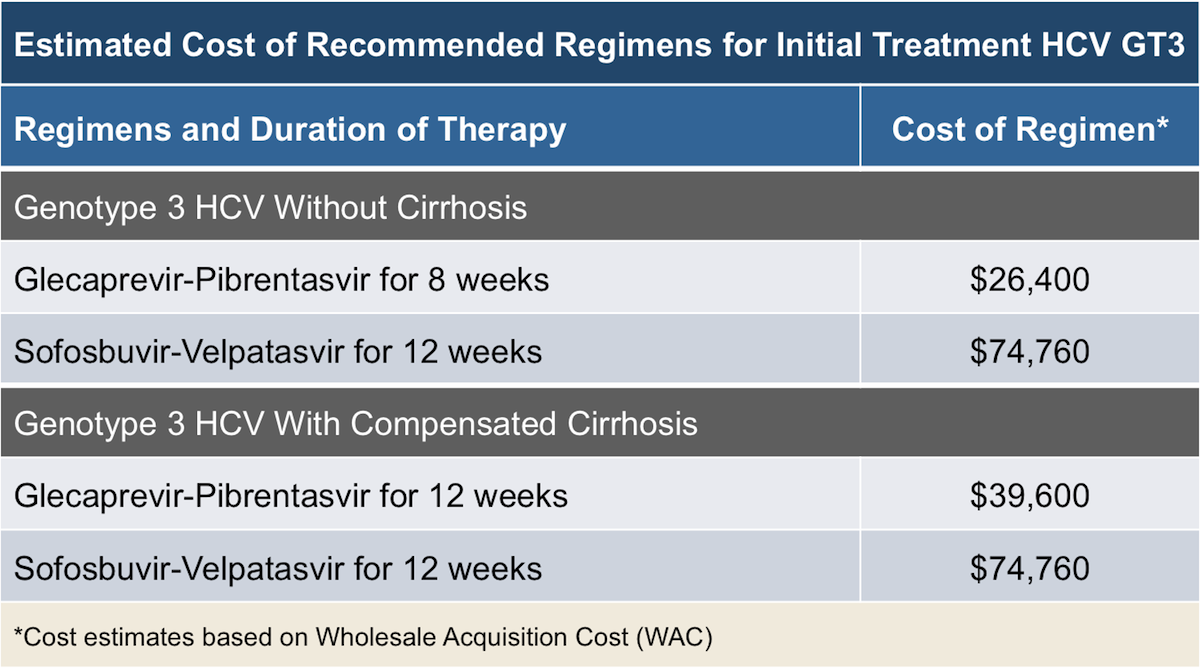
Treatment Of Hcv Genotype 3 With Compensated Cirrhosis: Sofosbuvir Plus Ribavirin
SOF plus RBV for 12 weeks is not recommended for treatment of cirrhotic patients with HCV genotype 3 infections. The overall SVR rates in naive cirrhotic patients treated for 12 weeks ranged from 21 to 34% . Two trials found that SOF plus RBV for 12 weeks for naive patients with cirrhosis resulted in SVR rates of 21% and 34% .
Sofosbuvir Plus And Ribavirin
The combination SOF plus PegIFN/RBV for 12 weeks is recommended by EASL and AASLD for the treatment of naive or treatment-experienced patients with compensated cirrhosis and HCV genotype 3 infection . This recommendation is based in only one study, which observed an overall SVR rate of 8692% in compensated cirrhotic patients .
What are the factors that affect the choice of treatment for HCV genotype 1?
For individuals with chronic HCV genotype 1 infection, the main factors that influence the choice and duration of therapy are cirrhosis status and prior treatment experience . With the use of certain regimens for persons with HCV genotype 1a, namely elbasvir-grazoprevir, the genotype 1 subtype (1a or 1b) also impacts the choice of therapy, as elbasvir-grazoprevir is only recommended for persons with HCV genotype 1a who do not have baseline NS5A resistance-associated substitutions (RASs). In addition, the HCV RNA level and the patient’s HIV status can impact the duration of ledipasvir-sofosbuvir, but does not affect the duration of other regimens. Finally, the cost of the regimen, insurance coverage, and provider preference can play a major role in the regimen choice. The following treatment recommendations are based on the AASLD-IDSA HCV Guidance for initial treatment of adults with HCV genotype 1 and for retreatment of adults in whom prior therapy failed, including those with HCV genotype 1. [ 4, 5]
What are the factors that influence the choice of treatment for HCV?
For treatment-naïve adults with chronic HCV genotype 1 infection, the main factors that influence the choice and duration of therapy are (1) presence or absence of cirrhosis, and (2) medication cost or insurance considerations. In the case of elbasvir-grazoprevir use, the HCV genotype 1 subtype (1a or 1b) is also important, as the presence of specific baseline NS5A RASs significantly reduces SVR12 rates in persons with HCV genotype 1a. [ 10, 11, 12] In cases where the genotype 1 subtype is not known, the individual should be treated as HCV genotype 1a. The baseline HCV RNA level generally does not influence the treatment choice or duration, except in treatment-naïve noncirrhotic patients in whom 8 or 12 weeks of ledipasvir-sofosbuvir is being considered. [ 13] Additional data from the HCV-TARGET registry and the Veterans Affairs National Healthcare System demonstrated comparable SVR rates of 94 to 98% for adults without cirrhosis treated with either 8 or 12 weeks of ledipasvir-sofosbuvir if the baseline HCV RNA levels were less than 6 million IU/mL. [ 14, 15, 16] In addition to the factors noted above, drug interactions may also influence the choice of therapy, particularly for individuals with HIV coinfection who are taking antiretroviral medications. Of note, individuals with HCV and HIV coinfection, depending on their specific antiretroviral therapy, are eligible for most of the same regimens for initial treatment of genotype 1 as for persons with HCV monoinfection, except that persons with HIV should not receive (1) any 8-week option of ledipasvir-sofosbuvir, or (2) the 8-week option of glecaprevir-pibrentasvir if cirrhosis is present. [ 11, 12, 17, 18]
What is genotype 1?
In the United States, genotype 1 hepatitis C virus (HCV) accounts for approximately 70 to 75% of all HCV infections. [ 1] . Accordingly, treatment of genotype 1 has the most extensive data and highest clinical relevance for hepatitis C treatment issues in the United States. In recent years, multiple studies using direct-acting antiviral (DAA) ...
What is the AASLD-IDSA HCV guidance?
The following is a summary of the AASLD-IDSA HCV Guidance for adults with HCV genotype 1 infection who are treatment experienced and failed prior DAA therapy , including those without cirrhosis and those with compensated cirrhosis. [ 5, 28, 29, 30] For individuals with cirrhosis, the AASLD-IDSA HCV Guidance defines compensated cirrhosis as Child-Turcotte-Pugh class A and decompensated cirrhosis as Child-Turcotte-Pugh class B or class C. The AASLD-IDSA HCV Guidance for retreatment is no longer genotype specific, but instead emphasizes a pangenotypic approach to retreatment based on the prior treatment regimen. In addition, the AASLD-IDSA HCV Guidance no longer includes recommendations for the retreatment of persons who experienced prior treatment failure with interferon-based therapy, including interferon plus first-generation protease inhibitors ( telaprevir, boceprevir ); these individuals have robust cure rates with modern DAA regimens similar to that observed with treatment-naïve persons. The recommended regimens in the tables below are based on prior regimen failure and listed by evidence level; when the evidence level is considered equivalent, the regimens are listed alphabetically.