
Heretofore, there are two delivery methods that have been applied to researches and have been proved to be feasible, including the inhalation of methane gas and injection with the methane-rich saline. This review studies on the clinical development of methane and discusses about the mechanism behind these protective effects.
Full Answer
How can we reduce methane emissions from wastewater treatment plants?
Methane emissions can be avoided, however, by treating the wastewater and the associated sludge under aerobic conditions or by capturing methane released under anaerobic conditions.
What is a methane project?
Methane is produced and emitted by landfills, during wastewater treatment, in natural gas and petroleum systems, from agricultural activities (livestock and rice cultivation), and during coal mining. Methane is basically ‘natural gas’ and can therefore be captured and used as a source of energy. There are two types of methane projects.
How do you get rid of methane in your body?
In addition to treating the overgrowth of archaea directly with one of the above treatments, those with methane often have specific symptoms that they need relief from: For bloating and gas, Gas-X, activated charcoal, and (my favorite) Atrantil are all recommended by Dr. Siebecker.
What is the best treatment for methane SIBO?
Dr. Siebecker recommends one of these treatments for methane SIBO: Rifaximin plus neomycin (requires a doctor’s prescription) Rifaximin plus metronidazole (requires a doctor’s prescription) Allicin (the active component of garlic, found in the supplement Allimed) plus one other herb: berberine, oregano, or neem
How is methane gas treated?
Methane SIBO is initially treated by working to reduce the overgrowth of bacteria in the small intestine. The treatment may involve a combination of dietary changes, antibiotic use, probiotics and prebiotics. It is also important that the underlying issue is addressed.
How do you remove methane?
Common clay materials may help curb methane emissions. With special treatment, minerals called zeolites — commonly found in cat litter — can efficiently remove the greenhouse gas from the air, researchers report.
How is methane recovered?
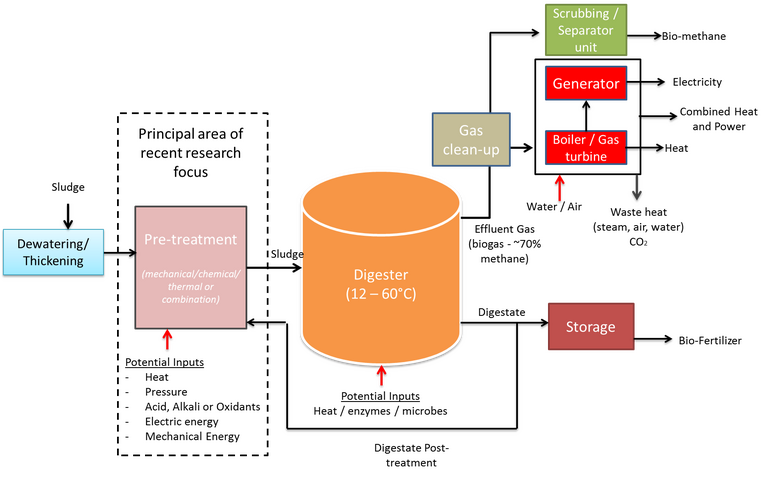
There are several techniques for the recovery of dissolved methane in the anaerobic wastewater treatment effluent. The most common techniques for these systems are aeration, gas stripping, and degassing membrane.
How can we reduce methane production?
Co-benefits to using feed additivesReduction of methane emissions through feed additives, such as fats and oils, can reduce methane production by about 18% and offer energy and protein to the animal. ... Reducing methane emissions is deemed 'additional' to normal management practices.
Can methane be removed from natural gas?
Natural gas, for instance, always contains quite a bit of carbon dioxide (the greenhouse gas CO2), sometimes up to 50 percent. To purify the methane -- or, in other words, remove the CO2 -- the industry often uses membranes. These membranes function as molecular sieves that separate the methane and the CO2.
Can methane be filtered?
Methane cannot be filtered. Unfortunately, methane is not just dangerous – it's also unfilterable. Adsorption and chemisorption, the two processes by which gas and odor air filters remove chemicals from the air, are ineffective against methane, which has an extremely low molecular weight.
How is biogas treated?
In general terms, biogas treatment is accomplished by physico-chemical methods, scrubbing being extensively used for H2S and CO2 removal. However, dilution (venting) has been an extensive disposal method in some small- and medium-size anaerobic plants treating municipal wastewaters.
What is methane gas recovery system?
The system makes electricity from methane. In turn, it can lower energy costs. It can also control odors and improve manure handling. The system can reduce potential for surface and groundwater contamination. It can also control harmful pathogens.
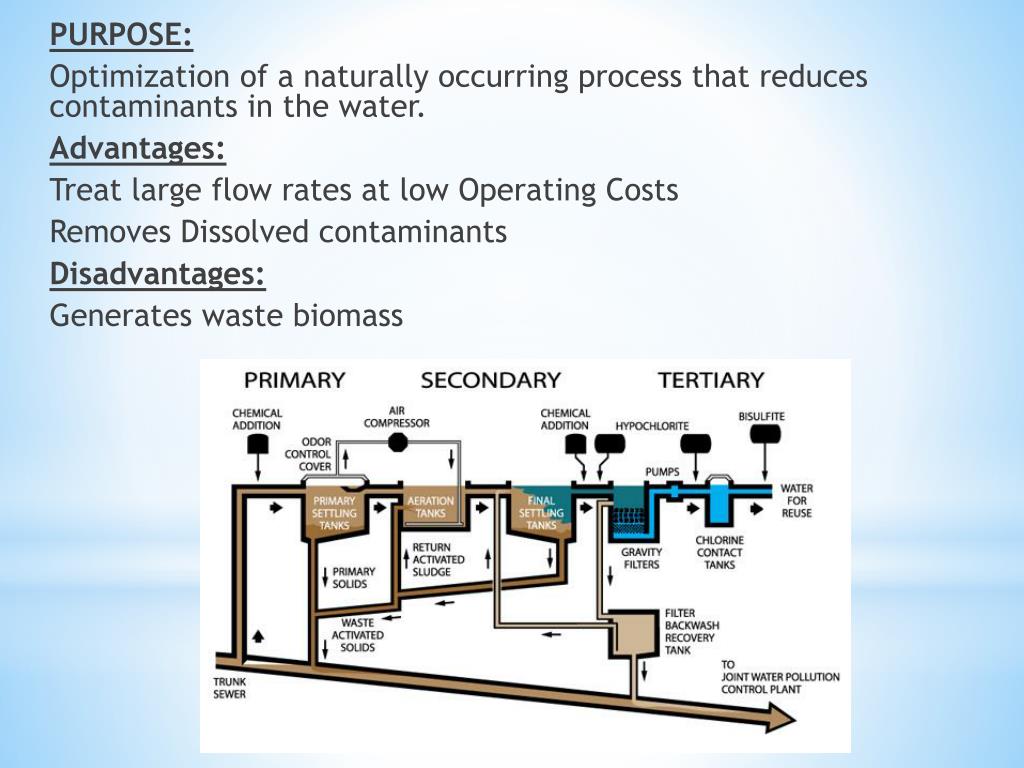
Does wastewater treatment produce methane?
Methane is emitted during the handling and treatment of municipal wastewater through the anaerobic decomposition of organic material. Most developed countries rely on centralized aerobic wastewater treatment systems to collect and treat municipal wastewater.
How can we reduce methane produced by cattle?
Cattle on carbohydrate -rich diets with high intake will produce less methane as a percentage of dietary gross energy. Grinding and pelleting of forages increases passage rate and reduces methane emitted by the animal.
How can we reduce methane in landfills?
Biosolids are used to create a special topsoil to cover decommissioned landfills. This topsoil contains microorganisms that convert methane into carbon dioxide, a much less potent greenhouse gas. This can reduce greenhouse gas emissions from landfills by as much as 95%.
Why is methane important?
As the major constituent of natural gas, methane is important for electricity generation by burning it as a fuel in a gas turbine or steam generator. Compared to other hydrocarbon fuels, methane produces less carbon dioxide for each unit of heat released.
Who discovered methane?
In November 1776, methane was first scientifically identified by Italian physicist Alessandro Volta in the marshes of Lake Maggiore straddling Italy and Switzerland. Volta was inspired to search for the substance after reading a paper written by Benjamin Franklin about "flammable air". Volta collected the gas rising from the marsh, and by 1778 had isolated the pure gas. He also demonstrated that the gas could be ignited with an electric spark.
How much heat does methane produce?
However, it produces more heat per mass (55.7 kJ/g) than any other organic molecule due to its relatively large content of hydrogen, which accounts for 55% of the heat of combustion but contributes only 25% of the molecular mass of methane. In many cities, methane is piped into homes for domestic heating and cooking.
What is the boiling point of methane?
Methane has a boiling point of −161.5 °C at a pressure of one atmosphere.
How many molecular orbitals does methane have?
Methane is a tetrahedral molecule with four equivalent C–H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H. The lowest-energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on ...
What is the chemical formula for methane?
Methane ( US: / ˈmɛθeɪn /, UK: / ˈmiːθeɪn /) is a chemical compound with the chemical formula CH4 (one atom of carbon and four atoms of hydrogen ). It is a group-14 hydride and the simplest alkane and is the main constituent of natural gas.
Why is partial oxidation of methane to methanol challenging?
Partial oxidation of methane to methanol is challenging because the reaction typically progresses all the way to carbon dioxide and water even with an insufficient supply of oxygen. The enzyme methane monooxygenase produces methanol from methane, but cannot be used for industrial-scale reactions. Some homogeneously catalyzed systems and heterogeneous systems have been developed, but all have significant drawbacks. These generally operate by generating protected products which are shielded from overoxidation. Examples include the Catalytica system, copper zeolites, and iron zeolites stabilizing the alpha-oxygen active site.
How does methane protect cells?
Methane, the simplest organic compound, was deemed to have little physiological action for decades. However, recently, many basic studies have discovered that methane has several important biological effects that can protect cells and organs from inflammation, oxidant, and apoptosis. Heretofore, there are two delivery methods that have been applied to researches and have been proved to be feasible, including the inhalation of methane gas and injection with the methane-rich saline. This review studies on the clinical development of methane and discusses about the mechanism behind these protective effects. As a new field in gas medicine, this study also comes up with some problems and prospects on methane and further studies.
Is methane a hotspot?
Methane, the most abundant organic compound on earth, was ignored in the medical field for decades. However, it has become a hotspot in therapeutic gas field. Recently, researchers have discovered some protective effect of methane and focus on the therapeutic function in I/R injury and inflammation disorder.
Is methane safe for rodents?
Nevertheless, methane should be used and stored with reliable tools and safety must always be the first concern. According to the study of Boros et al., the gas mixture of oxygen and methane (21% O 2 + 2.5% CH 4) is safe for rodents [ 8.
Is methane a non-toxic gas?
It is generally acknowledged that methane is a simple nontoxic asphyxiant, which means it is inherently nontoxic. Methane can be delivered via inhalation through many methods, including ventilator and facemask. As a flammable and explosive gas, the safe concentration of methane in pure oxygen is 4.9%. Nevertheless, methane should be used and stored with reliable tools and safety must always be the first concern. According to the study of Boros et al., the gas mixture of oxygen and methane (21% O 2 + 2.5% CH 4) is safe for rodents [ 8#N#M. Boros, M. Ghyczy, D. Érces et al., “The anti-inflammatory effects of methane,” Critical Care Medicine, vol. 40, no. 4, pp. 1269–1278, 2012. View at: Publisher Site | Google Scholar#N#See in References#N#].
Is methane a biological substance?
However, scientists recently reveal the biological effect of methane, especially the properties of anti-inflammatory, antioxidant, antiapoptosis and other clinic effects of methane, remains to be discovered.
Does methane poison the brain?
CO poison is an important cause of the accidental death. Methane protects brain from acute CO poisoning-induced injury with the properties of antioxidant, anti-inflammatory, and antiapoptotic. A finding suggested that methane reduced the level of inflammatory cytokines such as tumor necrosis factor- α (TNF- α) and interleukin1- β (IL-1 β) in the brain but had no effect on interleukin 6 (IL-6) expression. In addition, the oxidative products such as malondialdehyde (MDA), 3-nitrotyrosine (3-NT), and 8-hydroxydeoxyguanosine (8-OHdG) were reduced after methane treatment while the amount of SOD in the hippocampus and cortex was decreased, which improved neuronal injury [ 30#N#M. Shen, D. Fan, Y. Zang et al., “Neuroprotective effects of methane-rich saline on experimental acute carbon monoxide toxicity,” Journal of the Neurological Sciences, vol. 369, pp. 361–367, 2016. View at: Publisher Site | Google Scholar#N#See in References#N#].
What are some examples of methane projects?
Through combustion, methane gas is turned into less potent carbon dioxide and water. Examples of such projects include the capture and flaring of landfill gas and of coal mining gas.
Where is methane produced?
Methane is produced and emitted by landfills, during wastewater treatment, in natural gas and petroleum systems , from agricultural activities (livestock and rice cultivation), and during coal mining. Methane is basically ‘natural gas’ and can therefore be captured and used as a source of energy. There are two types of methane projects.
What are the effects of methane?
What are the potential health effects of methane? 1 Inhalation: Low concentrations are not harmful. A high concentration can displace oxygen in the air. If less oxygen is available to breathe, symptoms such as rapid breathing, rapid heart rate, clumsiness, emotional upsets and fatigue can result. As less oxygen becomes available, nausea and vomiting, collapse, convulsions, coma and death can occur. Symptoms occur more quickly with physical effort. Lack of oxygen can cause permanent damage to organs including the brain and heart. 2 Skin Contact: Not irritating. Direct contact with the liquefied gas can chill or freeze the skin (frostbite). Symptoms of mild frostbite include numbness, prickling and itching. Symptoms of more severe frostbite include a burning sensation and stiffness. The skin may become waxy white or yellow. Blistering, tissue death and infection may develop in severe cases. 3 Eye Contact: Not irritating. Direct contact with the liquefied gas can freeze the eye. Permanent eye damage or blindness can result. 4 Ingestion: Not a relevant route of exposure (gas). 5 Effects of Long-Term (Chronic) Exposure: Not harmful. 6 Carcinogenicity: Not a carcinogen.
How to prevent a fire?
Inhalation: Take precautions to prevent a fire (e.g. remove sources of ignition). In case of oxygen deficiency: take precautions to ensure your own safety before attempting rescue (e.g. wear appropriate protective equipment). Move victim to fresh air. Keep at rest in a position comfortable for breathing.
How to protect oxygen cylinders?
Secure cylinder in an up-right position. Protect cylinders from damage. Use a suitable hand truck to move cylinders; do not drag, roll, slide, or drop. Prevent accidental contact with incompatible chemicals.
How can methane emissions be reduced?
If flaring cannot feasibly be avoided, methane emissions can be reduced by improving the efficiency of the combustion in the flare. Since the design of a flare depends on the volume of and variations in gas flow, methods for improving combustion differ between low-volume and high-volume flares.
What is a synopsis of methane?
This Synopsis describes actions that an organisation can take to help manage methane emissions. Any actions or recommendations are not mandatory; they are simply one effective way to help manage methane emissions. Other approaches might be as effective, or more effective in a particular situation.
How to store gas that might otherwise be flared?
Storing gases that might otherwise be flared by injecting them into oil and gas reservoirs (which may also increase oil and gas production). Finding alternative uses for the gas, often to generate electricity. Improving the efficiency of flaring. Methods for reducing emissions from flaring have many elements in common with best practice ...
What can a gas turbine do?
Gas turbines and ‘reciprocating engines’ can convert gases that would otherwise be flared into electricity. The electricity can be used on-site to power equipment (including controllers, pumps and compressors) or sold to the grid.
How to recover waste gas?
Recover waste gases using vapor-recovery units. Vapor-recovery units can capture flash gas from tanks and compress it into the gas line so it can be sold rather than being released into the atmosphere or flared . Recover waste gases from well-testing and completion. After a new well is drilled, it is brought into production through in ...
Can natural gas be used to generate electricity?
If the waste gas cannot be recovered to be sold as a natural gas or natural-gas liquid product, or cannot be stored, it may be able to be used for generating electricity. If flaring cannot feasibly be prevented, improving the efficiency of flares can reduce emissions of methane. Methane emissions from flaring can be reduced in the following ways:
How can methane be avoided?
Methane emissions can be avoided, however, by treating the wastewater and the associated sludge under aerobic conditions or by capturing methane released under anaerobic conditions. Projects with technology that can capture methane from ...
What are some activities that release methane?
Fossil fuel production, rice cultivation, biomass burning, and waste management are some of the activities that release methane. In the case of organic waste, it is produced from microbial decomposition of organic matter in the absence of oxygen (Anaerobic decomposition).
What is methane emitted from?
Methane emission from waste water treatment plants can earn carbon revenue. Methane (CH 4) is emitted from both anthropogenic and natural sources. Fossil fuel production, rice cultivation, biomass burning, and waste management are some of the activities that release methane. In the case of organic waste, it is produced from microbial decomposition ...
Where is wastewater treated?
Wastewater from domestic (municipal sewage) and industrial sources are treated in municipal sewage treatment facilities and private effluent treatment plants (ETPs). If the wastewater contains loads of organic constituents (with high Chemical Oxygen Demand- COD) then it is treated anaerobically.
Best Available Technologies (BAT) for WtE in Developing Countries
Suani Teixeira Coelho, Rocio Diaz-Chavez, in Municipal Solid Waste Energy Conversion in Developing Countries, 2020
Worldwide Coalbed Gas Development
Indonesia is the fifth major coal producer of the world behind India, Australia, United Stats, and China, respectively ( BP, 2012 ). Continued growth of coal production during the past few years is attributed to demand from neighboring countries such as China, Japan, South Korea, and the Philippines ( USEPA, 2010 ).
Overburden response to longwall mining
Hua Guo, ... Deepak Adhikary, in Advances in Coal Mine Ground Control, 2017
Vision 2023: Assessing the feasibility of electricity and biogas production from municipal solid waste in Turkey
Methane capture and combustion at the landfills does not only provide economic return but also help to tackle climate change due to greenhouse gas emission savings. Complete combustion of 1 mol of methane yields in generation of 1 mol carbon dioxide and 1 mol water vapour.
Formation and impact of granules in fostering clean energy production and wastewater treatment in upflow anaerobic sludge blanket (UASB) reactors
Anaerobic reactors have acquired a new relevance in recent years due to their ability to generate methane from biodegradable wastewaters—thereby producing clean energy.
Methane capture from livestock manure
S.M. Tauseef, ... S.A. Abbasi, in Journal of Environmental Management, 2013
Coal mine methane: A review of capture and utilization practices with benefits to mining safety and to greenhouse gas reduction
C. Özgen Karacan, ... Sally Phipps, in International Journal of Coal Geology, 2011
What is methylamphetamine used for?
Methamphetamine (contracted from N- methylamphetamine) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder and obesity. Methamphetamine was discovered in 1893 and exists as two enantiomers: levo-methamphetamine ...
Where is meth produced?
The Golden Triangle (Southeast Asia), specifically Shan State, Myanmar, is the world's leading producer of methamphetamine as production has shifted to Yaba and crystalline methamphetamine, including for export to the United States and across East and Southeast Asia and the Pacific.
How does methamphetamine affect the liver?
Methamphetamine is metabolized by the liver enzyme CYP2D6, so CYP2D6 inhibitors will prolong the elimination half-life of methamphetamine. Methamphetamine also interacts with monoamine oxidase inhibitors (MAOIs), since both MAOIs and methamphetamine increase plasma catecholamines; therefore, concurrent use of both is dangerous. Methamphetamine may decrease the effects of sedatives and depressants and increase the effects of antidepressants and other stimulants as well. Methamphetamine may counteract the effects of antihypertensives and antipsychotics due to its effects on the cardiovascular system and cognition respectively. The pH of gastrointestinal content and urine affects the absorption and excretion of methamphetamine. Specifically, acidic substances will reduce the absorption of methamphetamine and increase urinary excretion, while alkaline substances do the opposite. Due to the effect pH has on absorption, proton pump inhibitors, which reduce gastric acid, are known to interact with methamphetamine.
How much meth is excreted in urine?
Methamphetamine is excreted by the kidneys, with the rate of excretion into the urine heavily influenced by urinary pH. When taken orally, 30–54% of the dose is excreted in urine as methamphetamine and 10–23% as amphetamine. Following IV doses, about 45% is excreted as methamphetamine and 7% as amphetamine.
How long does it take for meth to be absorbed into the blood?
Following oral administration, methamphetamine is well-absorbed into the bloodstream, with peak plasma methamphetamine concentrations achieved in approximately 3.13–6.3 hours post ingestion. Methamphetamine is also well absorbed following inhalation and following intranasal administration. Due to the high lipophilicity of methamphetamine, it can readily move through the blood–brain barrier faster than other stimulants, where it is more resistant to degradation by monoamine oxidase. The amphetamine metabolite peaks at 10–24 hours. Methamphetamine is excreted by the kidneys, with the rate of excretion into the urine heavily influenced by urinary pH. When taken orally, 30–54% of the dose is excreted in urine as methamphetamine and 10–23% as amphetamine. Following IV doses, about 45% is excreted as methamphetamine and 7% as amphetamine. The half-life of methamphetamine is variable with a range of 5–30 hours.
What are the psychological effects of methamphetamine?
The psychological effects of methamphetamine can include euphoria, dyspho ria, changes in libido, alertness, apprehension and concentration, decreased sense of fatigue, insomnia or wakefulness, self-confidence, sociability, irritability, restlessness, grandiosity and repetitive and obsessive behaviors. Peculiar to methamphetamine and related stimulants is " punding ", persistent non-goal-directed repetitive activity. Methamphetamine use also has a high association with anxiety, depression, amphetamine psychosis, suicide, and violent behaviors.
What are the effects of methamphetamine?
The physical effects of methamphetamine can include loss of appetite, hyperactivity, dilated pupils, flushed skin, excessive sweating, increased movement, dry mouth and teeth grinding (leading to " meth mouth "), headache, irregular heartbeat (usually as accelerated heartbeat or slowed heartbeat ), rapid breathing, high blood pressure, low blood pressure, high body temperature, diarrhea, constipation, blurred vision, dizziness, twitching, numbness, tremors, dry skin, acne, and pale appearance. Long-term meth users may have sores on their skin; these may be caused by scratching due to itchiness or the belief that insects are crawling under their skin, and the damage is compounded by poor diet and hygiene. Numerous deaths related to methamphetamine overdoses have also been reported as well.
Properties and Bonding
Chemical Reactions
- The primary chemical reactions of methane are combustion, steam reforming to syngas, and halogenation. In general, methane reactions are difficult to control.
Uses
- Methane is used in industrial chemical processes and may be transported as a refrigerated liquid (liquefied natural gas, or LNG). While leaks from a refrigerated liquid container are initially heavier than air due to the increased density of the cold gas, the gas at ambient temperature is lighter than air. Gas pipelinesdistribute large amounts of natural gas, of which methane is the principal …
Generation
- Geological routes
The two main routes for geological methane generation are (i) organic (thermally generated, or thermogenic) and (ii) inorganic (abiotic). Thermogenic methane occurs due to the breakup of organic matter at elevated temperatures and pressures in deep sedimentary strata. Most metha… - Biological routes
Most of Earth's methane is biogenic and is produced by methanogenesis, a form of anaerobic respiration only known to be conducted by some members of the domain Archaea. Methanogens occupy landfills and other soils, ruminants (for example, cattle), the guts of termites, and the an…
Occurrence
- Methane was discovered and isolated by Alessandro Volta between 1776 and 1778 when studying marsh gas from Lake Maggiore. It is the major component of natural gas, about 87% by volume. The major source of methane is extraction from geological deposits known as natural gas fields, with coal seam gas extraction becoming a major source (see coal bed methane extra…
History
- In November 1776, methane was first scientifically identified by Italian physicist Alessandro Volta in the marshes of Lake Maggiore straddling Italy and Switzerland. Volta was inspired to search for the substance after reading a paper written by Benjamin Franklin about "flammable air". Volta collected the gas rising from the marsh, and by 1778 had isolated pure methane.He also demon…
Safety
- Methane is nontoxic, yet it is extremely flammable and may form explosive mixtures with air. Methane is also an asphyxiant if the oxygen concentration is reduced to below about 16% by displacement, as most people can tolerate a reduction from 21% to 16% without ill effects. The concentration of methane at which asphyxiation risk becomes significant is much higher than th…
Cited Sources
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
External Links
- Methane at The Periodic Table of Videos(University of Nottingham)
- International Chemical Safety Card 0291
- Gas (Methane) Hydrates – A New Frontier– United States Geological Survey
- Lunsford, Jack H. (2000). "Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century". Catalysis Today. 63 (2–4): 165–174. doi:10.1016/S0…
- Methane at The Periodic Table of Videos(University of Nottingham)
- International Chemical Safety Card 0291
- Gas (Methane) Hydrates – A New Frontier– United States Geological Survey
- Lunsford, Jack H. (2000). "Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century". Catalysis Today. 63 (2–4): 165–174. doi:10.1016/S0920-5861(00)00456-9.
Abstract
- Methane, the simplest organic compound, was deemed to have little physiological action for decades. However, recently, many basic studies have discovered that methane has several important biological effects that can protect cells and organs from inflammation, oxidant, and apoptosis. Heretofore, there are two delivery methods that have been applied to researches and …
Introduction
- Methane, the simplest alkane, is the most plentiful organics on earth and has been studied for hundreds of years since its discovery in 1778. Being the main component of natural gas, methane is used as gas fuel. In past decades, it has been proven to be related to global warming since it contributes 20% of the greenhouse gases in the atmosphere and the concentration has raised ra…
Delivery of Methane
- It is generally acknowledged that methane is a simple nontoxic asphyxiant, which means it is inherently nontoxic. Methane can be delivered via inhalation through many methods, including ventilator and facemask. As a flammable and explosive gas, the safe concentration of methane in pure oxygen is 4.9%. Nevertheless, methane should be used and stored...
The Biological Effects of Methane
- Acute liver failure (ALF) is the clinical manifestation of sudden and severe hepatic injury [12]. In the United States, over 6% of liver-related mortality was caused by ALF in 2005 [13]. Necrosis and apoptosis of hepatocytes induced by infection, chemical, or biological toxins are the dominant pathological causes of acute liver failure [13–15]. In a carbon tetrachloride- (CCl4-) induced acut…
Discussion
- Scientists have already revealed a few biological properties of methane in inflammation, oxidative stress, and apoptosis. Through these properties, methane can influence several pathological processes including I/R injury and sepsis. What is the exact mechanism underlying the protective properties of methane? The answer is unclear. So far, different researchers have come up with d…
Conflicts of Interest
- The authors declare that there is no conflict of interest regarding the publication of this article.
Authors’ Contributions
- Yifan Jia participated in the research design and writing the paper. Zeyu Li participated in the literature review and writing the paper. They contributed equally to the paper. Chang Liu and Jingyao Zhang provided substantial advice in designing the study and revising the paper. Yifan Jia and Zeyu Li contribute equally to the paper.
Acknowledgments
- The authors are indebted to all individuals who participated in or helped with this research project. This study was supported by funding from “the National Nature Science Foundation of China” (Grant no. 81601672), “the Project of Innovative Research Team for Key Science and Technology in Shaanxi province” (Grant no. 2013KCJ-23), and “the Fundamental Research Funds for the Cent…