Is HCV treatment cost-effective?
However, while Sovaldi’s wholesale acquisition cost (WAC) is listed for $84,000 and Harvoni’s at $94,500 for a 12-week treatment, the average revenue per treatment reported by Gilead in 2015, including both 12- and 24-week-long treatments, was estimated to be around $54,000 and during the first 6 months of 2016 the price per treatment dropped another 22% — a possible response …
How does Medicaid pay for HCV treatment?
Jun 11, 2021 · Analysis of prescription drugs for the treatment of hepatitis C in the United States. Sovaldi, a direct-acting antiviral (DAA) treatment for hepatitis C virus (HCV), had an average wholesale acquisition cost (WAC) of $1,000 per day in …
How do pharmaceutical companies price HCV drugs?
When using WAC as the primary drug price input, the 2018 HCV treatment option was still preferred to other scenarios . Depending on the decision maker’s willingness-to-pay threshold ($50,000/QALY, $100,000/QALY, and $150,000/QALY), the final determination of cost-effectiveness may have changed.
How are HCV DAA regimens compared to cost-effectiveness?
The wholesale acquisition cost (WAC) for a 12-week treatment course with Viekira Pak is $83,319. The cost for a 24-treatment course with the Viekira Pak is $166,638. The exact added cost of ribavirin, if used, is more difficult to determine because of variable daily doses when using the recommended weight-based dosing and the availability of ribavirin through multiple …

What is the cost of HCV treatment?
The cost of hep C treatment varies depending on the type of drug. However, an 8- to 12-week course can range from $54,000 to $95,000 (or higher). For example, the price of a 12-week course of Zepatier can be as much as $54,600, and a 12-week course of Harvoni can cost as much as $94,500.Sep 2, 2021
What are the four drugs used to treat HCV?
Here are the medications available to treat hepatitis C, plus some helpful information about what to expect with their treatment.Ribavirin. ... Direct-acting antivirals (DAAs) ... Combination drugs. ... Ledipasvir-sofosbuvir (Harvoni) ... Elbasvir-grazoprevir (Zepatier) ... Sofosbuvir-velpatasvir (Epclusa)More items...
How much do direct-acting antivirals cost?
Conclusions: Within the next 15 years, large-scale manufacture of 2 or 3 drug combinations of HCV DAAs is feasible, with minimum target prices of $100-$250 per 12-week treatment course. These low prices could make widespread access to HCV treatment in low- and middle-income countries a realistic goal.
WHO guidelines HCV treatment?
WHO recommends therapy with pan-genotypic direct-acting antivirals (DAAs) for persons over the age of 12 years. DAAs can cure most persons with HCV infection, and treatment duration is short (usually 12 to 24 weeks), depending on the absence or presence of cirrhosis.Jul 27, 2021
What is the best hep C drug?
Hepatitis C is treated using direct-acting antiviral (DAA) tablets. DAA tablets are the safest and most effective medicines for treating hepatitis C. They're highly effective at clearing the infection in more than 90% of people.
What is the latest treatment for hep C?
The new hepatitis C treatments are sofosbuvir with ledipasvir (Harvoni); sofosbuvir (Sovaldi); daclatasvir (Daklinza); and ribavirin (Ibavyr). These new treatments are now available on the Pharmaceuticals Benefits Scheme.Mar 1, 2016
How much is hep C treatment in India?
The generic version of these drugs are available in cities such as Bengaluru Hyderabad and Chennai at the cost of Rs70000 or around $1000 USD for the entire treatment regimen.
What is DAA treatment?
Importance Direct-acting antiviral (DAA) drugs are highly effective in curing hepatitis C virus (HCV) infection. Previous simulations showed extended life as a key health advantage of DAA drugs, but real-world evidence on the association between DAA treatment and reduced mortality is limited.Jul 21, 2020
How do direct-acting antivirals work?
Direct-acting antivirals work by blocking the action of proteins which are essential for making new hepatitis C viruses.
How long does Hep C take to damage liver?
After many years some people will have minimal liver damage with no scarring while others can progress to cirrhosis (extensive scarring of the liver) within less than ten years. On average it takes about twenty years for significant liver scarring to develop.
How long is Hep C treatment?
How long is the treatment? Treatment is usually 8-12 weeks long but can be as much as 16 weeks long in certain situations. Some patients with more damage to their liver may require 24 weeks of treatment, but this is uncommon. The duration depends on the medication, and specific HCV factors in particular patients.
What is the difference between hepatitis AB and C?
The most significant difference between hepatitis B and hepatitis C is that people may get hepatitis B from contact with the bodily fluids of a person who has the infection. Hepatitis C usually only spreads through blood-to-blood contact.Oct 25, 2018
What is cost effectiveness analysis?
Cost-effectiveness analysis (CEA) compares the relative costs and outcomes of 2 or more interventions. CEA explicitly recognizes budget limitations for healthcare spending and seeks to maximize public health benefits within those budgetary constraints. The core question that CEA addresses is whether to invest limited healthcare dollars in a new treatment/therapy or use that money to invest in another healthcare intervention that would provide better outcomes for the same monetary investment. The focus of CEA is, therefore, not simply cost or saving money but health benefits. It assumes that all available resources will be spent and provides a framework for prioritizing among available treatment options by formally assessing the comparative costs and health benefits accrued from a new treatment relative to current treatment.
What is the time horizon for CEA?
From a societal perspective, CEA uses a lifetime time horizon, meaning it considers lifetime costs and benefits, including those that occur in the distant future. Business budget planning, however, typically assumes a 1-year to 5-year perspective.
What does private insurance do?
Private insurance companies often have separate pharmacy and medical budgets, and use PBMs or directly negotiate drug pricing with pharmaceutical companies. Insurance companies determine formulary placement, which impacts the choice of regimens and out-of-pocket expenses for patients.
Is life expectancy a measure of benefit?
Life expectancy is a valuable measure of benefit but considering only mortality benefits fails to recognize the value of treatments that improve quality of life. The quality-adjusted life-year (QALY) provides a measure that integrates both longevity and quality of life and is the preferred outcome for CEA.
Is an intervention cost effective?
An intervention that is cost-effective is not necessarily affordable. Affordability refers to whether a payer has sufficient resources in its annual budget to pay for a new therapy for all who might need or want it within that year . Several characteristics of CEA limit its ability to speak to the budgetary impact of interventions being implemented in the real world.
Is HCV cost effective?
There is no formula that provides a good means of integrating the concerns of value and affordability. When new HCV therapies are deemed cost-effective, it indicates that these therapies provide good benefit for the resources invested and providing such therapy to more people would be a good long-term investment.
Is routine HCV testing cost effective?
Generally, routine HC V testing is cost-effective because the incidence and prevalence of HCV remain high in people who inject drugs with a notable rising prevalence in young adults who may not readily report their stigmatized risk behaviors.
What is the primary analysis for this methodology study focused on?
The primary analysis for this methodology study focused on the changing costs and effectiveness estimates at each time point to estimate incremental cost-effectiveness ratios. A scenario analysis was conducted using only the WAC for each drug referenced in RED BOOK to describe the effect of using list versus net price in the CEA. 21
Is HCV treatment effective?
Treatment effectiveness for HCV has increased steadily, while treatment costs increased substantially from 2010-2014 before decreasing to its lowest point in 2018. The dynamic nature of CEAs in a disease state with rapid pharmaceutical innovation may cause some concern for decision makers who rely on a single analysis over time. Model transparency along with resources to update or revise model assumptions would enable organizations to provide more up-to-date results to inform formulary decisions.
When was ombitasvir approved?
On December 19, 2014, the United States FDA approved ombitasvir-paritaprevir-ritonavir and dasabuvir for the treatment of genotype 1 chronic hepatitis C infection in adults, including patients with compensated cirrhosis. The medications ombitasvir, paritaprevir, and dasabuvir do not have FDA approval for use as individual drugs outside ...
What is a Viekira pak?
The Viekira Pak is an all-oral regimen comprised of four medications: ombitasvir, paritaprevir, ritonavir, and dasabuvir. This regimen can be used with or without ribavirin. In the Viekira Pak, the ombitasvir-paritaprevir-ritonavir are combined as a fixed-dose tablet and the dasabuvir is a separate tablet. Ombitasvir is a NS5A inhibitor ...
How long does ombitasvir last?
Genotype 1b, with cirrhosis: ombitasvir-paritaprevir-ritonavir and dasabuvir plus ribavirin for 12 weeks. For patients with unknown genotype 1 subtype or mixed genotype 1 infection, use the regimen and duration as recommended for genotype 1a. For patients with HIV coinfection, use the same dosage recommendations as listed above.
Can Viekira Pak cause liver damage?
On October 22, 2015 the United States FDA issued a Drug Safety Warning that treatment with ombitasvir-paritaprevir-ritonavir and dasabuvir ( Viekira Pak) can cause serious liver injury, mostly in patients with underlying advanced liver disease. In most of the reported cases, the liver injury occurred within 1 to 4 weeks of starting treatment.
How many people in the US have HCV?
More than 3 million Americans are infected with HCV, with its prevalence concentrated among baby boomers, who were born between 1945 and 1965. 7 HCV causes more deaths in the United States than HIV/AIDS. 8 Chronic HCV is a cause of serious and costly liver diseases, such as cirrhosis and liver cancer, and related hospitalizations and costs have increased during the past decade. 9 Although the burden of HCV can be reduced through screening and treatments, the implementation of recommended screening is limited, and half of the infected population goes undiagnosed. 9
What drugs did Part D cover?
All Part D plans covered 2 new HCV drugs, Olysio and Sovaldi, and 98% of plans covered Harvoni ( ). Only 33% of MAPDs and 30% of PDPs covered Viekira Pak. Nearly every plan that covered these new drugs used prior authorization and nearly half of the plans used quantity limits. Almost all plans placed new HCV agents in a specialty tier and required coinsurance rather than co-payment. The average coinsurance rate was slightly higher among MAPDs than PDPs (31.4% vs 28.7%), but it varied more among MAPDs (20%-50%) than PDPs (25%-33%).
What is Medicare Part D?
Medicare Part D provides outpatient prescription drug coverage to the elderly and disabled. It is delivered through private plans, including standalone prescription drug plans (PDPs) or Medicare Advantage plans with prescription drug coverage (MA-PDs). Medicare specifies a standard Part D benefit package, but plans can modify the benefits as long as their schemes are equal in value to the standard package.
Does Part D insurance cover HCV?
Part D plans charge relatively high coinsurance for new HCV drugs, and they require rigorous utilization management, including prior authorization and quantity limits for those drugs. Little variation in coverage exists across plans, leaving few options for beneficiaries to choose a plan with better benefits.
Who Is NOT Eligible for Simplified Treatment
Current or prior episode of decompensated cirrhosis, defined as Child-Turcotte-Pugh (CTP) score ≥7 (ascites, hepatic encephalopathy, total bilirubin >2.0 mg/dL, albumin ≤3.5 g/dL, or INR ≥1.7)
Who Is Eligible for Simplified Treatment
Adults with chronic hepatitis C (any genotype) who have compensated cirrhosis (Child-Pugh A) and have not previously received hepatitis C treatment
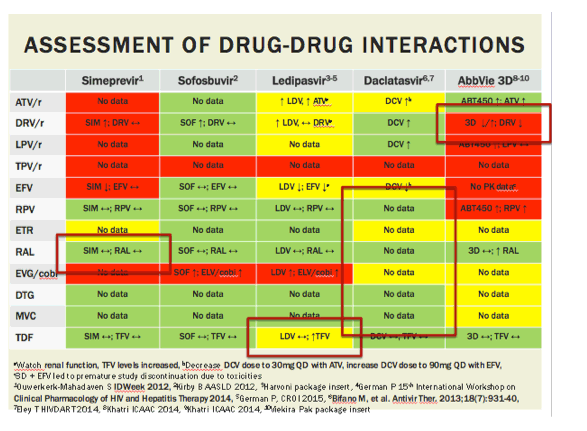
Drug Cost and Reimbursement
- Many organizations are involved with hepatitis C drug distribution and each can impact costs as well as decisions about which regimens are reimbursed (US GAO, 2015); (US CBO, 2015). The roles these organizations have in determining the actual price paid for drugs and who has access to treatment include the following: 1. Pharmaceutical companies determine the wholesale acqui…
Cost-Effectiveness
- Cost-effectiveness analysis (CEA) compares the relative costs and outcomes of 2 or more interventions. CEA explicitly recognizes budget limitations for healthcare spending and seeks to maximize public health benefits within those budgetary constraints. The core question that CEA addresses is whether to invest limited healthcare dollars in a new treatment/therapy or use that …
Affordability
- An intervention that is cost-effective is not necessarily affordable. Affordability refers to whether a payer has sufficient resources in its annual budget to pay for a new therapy for all who might need or want it within that year. Several characteristics of CEA limit its ability to speak to the budgetary impact of interventions being implemented in the real world. 1. Perspective on cost CEA seeks t…
Cost vs Affordability For HCV Treatment
- Despite a growing body of evidence that HCV treatment is cost-effective and may even be cost saving over the long term in some cases, many US payers—especially those offering Medicaid insurance products—continue to limit access to HCV treatment. Access has improved as cost has decreased but limitations remain. Proposed reductions in healthcare spending for Medicaid wou…
Cost-Effectiveness of Screening For HCV
- Several cost-effectiveness studies demonstrate that routine, one-time testing for HCV among all adults in the US would likely identify a substantial number of cases of HCV that are currently being missed, and that doing so would be cost-effective. One study employed simulation modeling to compare several versions of routine guidance, including routine testing for adults over the ages …
Conclusions
- Many studies have demonstrated the economic value of HCV screening (Chaillon, 2019); (Eckman, 2019); (Tasillo, 2019); (Assoumou, 2018); (Barocas, 2018); (Schackman, 2018); (Schechter-Perkins, 2018); (Lyons, 2016); (Hsieh, 2016); (Schackman, 2015) and treatment (Goel, 2018); (Chhatwal, 2017); (He, 2017); (Chahal, 2016); (Chhatwal, 2015); (Chidi, 2016); (Martin, 201…