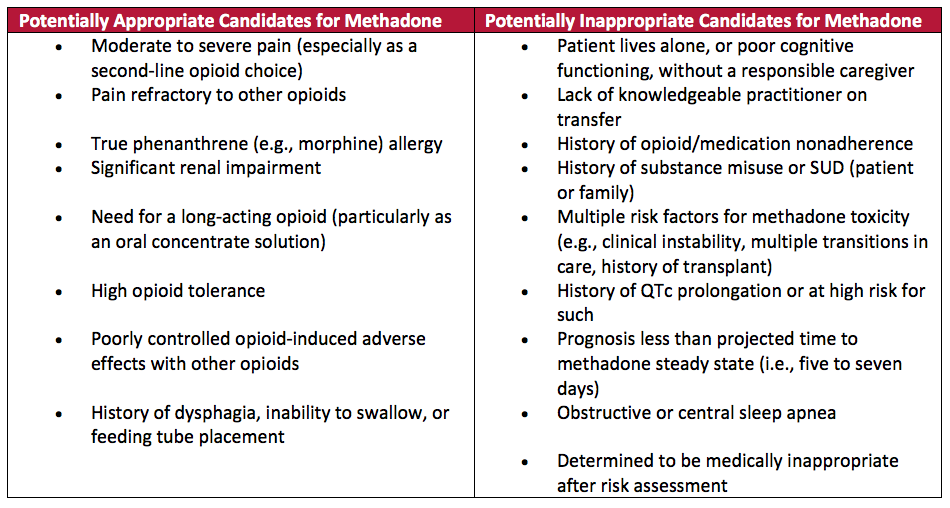
What is the maximum dose of morphine for palliative care?
5 rows · Aug 02, 2012 · The European Association for Palliative Care (EAPC) recommends one-sixth of the total 24-hr ...
When is it necessary to increase dosing of opioids in palliative care?
Feb 12, 2021 · Immediate-release morphine can also be initiated in an opioid-naïve patient using a regular immediate-release preparation and an ‘as required’ dose (e.g. BNF and NICE guidance recommend starting at 20–30mg in divided doses, which could, for example, be given as 5mg morphine sulphate immediate-release solution every four hours with 5mg immediate-release …
How much palliative care dose should I take?
The 2012 European Association of Palliative Care (EAPC) guidelines state that when paracetamol or NSAIDs are insuffi-cient, the addition of any step 2 weak opioid may achieve good pain relief or that a low-dose step 3 opioid (eg 30mg morphine or 20mg oxycodone in 24 hours) may be used instead. 3 The Palliative Care Formulary (PCF6) recommends that
What are the guidelines for the management of opioid toxicity?
Opioids in palliative care: full guideline (May 2012) Page 7 of 85 1 Recommendations Communication 1.1.1 When offering pain treatment with strong opioids to a patient with advanced and progressive disease, ask them about concerns such as: addiction tolerance side effects fears that treatment implies the final stages of life.

What is opioid use in palliative care?
Opioid use in palliative care: selection, initiation and optimisation. Opioids are commonly administered as part of end-of-life care. Pharmacists should know how to select, initiate and optimise opioids to best meet patient needs.
What does "palliative care" mean?
The World Health Organization (WHO) uses the term ‘palliative care’ to describe an approach to improving the quality of life of patients facing life-threatening illness through management of symptoms, such as pain [4] .
How are opioids metabolized?
Opioids are metabolised by cytochrome P450 (CYP450) enzymes and are consequently subject to drug-drug interactions mediated by enzyme induction or inhibition (see Table 2) [24]#N#. When selecting an opioid for initiation, the presence of interacting drugs should be considered. For example, clarithromycin can increase serum levels of oxycodone through inhibition of CYP3A4; therefore, initiating oxycodone when a patient is receiving clarithromycin risks elevated serum levels and causing adverse effects [25]#N#. Once the antibiotic course has finished, serum levels may decline and pain may be precipitated. Such variation in serum opioid levels may be difficult to manage, particularly in an outpatient setting. Therefore, it may be prudent to choose another opioid that does not interact (e.g. morphine).
What are the disadvantages of step 2 opioids?
Unlike strong opioids, another disadvantage of step 2 weak opioids is the analgesic ‘ceiling’ effect — where dose escalation beyond a certain level does not confer any additional analgesic benefit [8] . Moreover, most of the analgesic effect of the weak opioid codeine is gathered from its O-demethylation into morphine [9] .
What is the 3 step analgesic ladder?
In 1986, WHO proposed a three-step analgesic ladder for managing cancer pain, beginning with a non-opioid (with or without an adjuvant) analgesic as step 1 , escalating to a weak opioid (with or without an adjuvant, with or without a non-opioid) as step 2 if pain persists or worsens and , finally , advocating a strong opioid in moderate-to-severe pain as step 3 (with or without an adjuvant, with or without a non-opioid) [4]#N#. Regular administration of oral analgesic preparations — titrated according to response and benefit — in individuals who are monitored and managed for adverse effects, and provided with additional analgesia on an ‘as required’ basis, represent the guiding principles of cancer pain management [6]#N#.
Which organs are responsible for the excretion of opioids?
For most opioids, the major route of excretion is through the kidneys; however, for this to occur, hepatic metabolism is required to produce derivatives with greater water solubility [12] , [13] . Therefore, impairment in one or both of these organs can significantly reduce opioid clearance and lead to accumulation.
Can morphine be used for cirrhosis?
Consequently, morphine can be used, albeit cautiously, with gradual dose titration and increased dosing intervals, in patients with cirrhosis (see Table 1) [12] , [13] , [14]
Which is the strongest opioid for pain in palliative care?
Oral morphine is generally the first-line strong opioid for pain in palliative care. 2 Morphine is the most extensively studied, widely available and commonly used opioid in palliative care.
How long can you supply opioids?
Pharmacies can generally only supply strong opioids at a maximum of ten-day quantities for subsidy purposes, however, a patient with problems of mobility or access to a pharmacy can sign a declaration at the pharmacy to have the 30 day supply dispensed all at once.
How much Oxycodone is equivalent to 20 mg?
The oral availability of oxycodone is approximately twice that of morphine, therefore 20 mg oral morphine is approximately equivalent to 10 mg oral oxycodone. 10 In practice the conversion appears to be less than 2:1 for patients in palliative care, and it is important to review efficacy.
How long does fentanyl last?
Fentanyl patches should be removed after 72 hours, and not changed more frequently than this. Once fentanyl patches are discontinued residual medicine in the dermis will continue to have an effect for up to 24 hours, and the patient should be monitored for up to 48 hours for residual effects.
What is the most common symptom of palliative care in New Zealand?
Pain is estimated to be the most prevalent symptom preceding all deaths occurring in a palliative care setting in New Zealand. 1 Strong opioids, particularly morphine, are an effective treatment for moderate to severe pain , and as many as two-thirds of adults with terminal cancer will require treatment with a strong opioid. 2 A similar need for opioids is also observed in patients with other advanced and progressive illnesses, e.g. heart failure, kidney and liver disease, and neurodegenerative conditions. 2 Pain is increasingly regarded as the fifth vital sign and all patients in palliative care should be carefully assessed for pain to prevent under-treatment and reduced quality of life.
When can fentanyl be used as needed?
When a patient's renal function is significantly impaired, occasional doses of immediate release opioids can be used "as needed" until the fentanyl is providing analgesia, as well as being available for breakthrough pain during this time.
What are the features of pain assessment?
If a patient is experiencing cognitive decline or impairment it may be necessary to include behavioural features in the assessment of pain, e.g. breathing patterns, facial expressions or vocalisation. There are many tools available for assessing pain in people with cognitive decline or impaired speech.
How many people are treated with opioids?
An estimated 11% of adults experience daily pain. Millions of Americans are treated with prescription opioids for chronic pain. Primary care providers are concerned about patient addiction and report insufficient training in prescribing opioids.
What is the CDC's opioid prescribing guideline?
About CDC’s Opioid Prescribing Guideline. Improving the way opioids are prescribed through clinical practice guidelines can ensure patients have access to safer, more effective chronic pain treatment while reducing the number of people who misuse or overdose from these drugs. CDC developed and published the CDC Guideline for Prescribing Opioids ...
How much hydrocodone is in a tablet?
For example, tablets containing hydrocodone 5 mg and acetaminophen 300 mg taken four times a day would contain a total of 20 mg of hydrocodone daily, equivalent to 20 MME daily; extended-release tablets containing oxycodone 10mg and taken twice a day would contain a total of 20mg of oxycodone daily, equivalent to 30 MME daily.
How many people use opioids in 2016?
Improving the way opioids are prescribed through clinical practice guidelines can ensure patients have access to safer, more effective chronic pain treatment while reducing the risk of opioid use disorder, overdose, and death. More than 11.5 million Americans, aged 12 or older, reported misusing prescription opioids in 2016. 1
How long does pain last with opioids?
Recommendations focus on the use of opioids in treating chronic pain (pain lasting longer than 3 months or past the time of normal tissue healing) outside of active cancer treatment, palliative care, and end-of-life care.
Can conversion factors be used to benchmark against MME dosage thresholds?
Conversion factors for drugs prescribed or provided as part of medication-assisted treatment for opioid use disorder should not be used to benchmark against MME dosage thresholds meant for opioids prescribed for pain.
What is the CDC guideline for opioids?
This guideline provides recommendations for primary care clinicians who are prescribing opioids for chronic pain outside of active cancer treatment, palliative care, and end-of-life care. The guideline addresses 1) when to initiate or continue opioids for chronic pain; 2) opioid selection, dosage, duration, follow-up, and discontinuation; and 3) assessing risk and addressing harms of opioid use. CDC developed the guideline using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework, and recommendations are made on the basis of a systematic review of the scientific evidence while considering benefits and harms, values and preferences, and resource allocation. CDC obtained input from experts, stakeholders, the public, peer reviewers, and a federally chartered advisory committee. It is important that patients receive appropriate pain treatment with careful consideration of the benefits and risks of treatment options. This guideline is intended to improve communication between clinicians and patients about the risks and benefits of opioid therapy for chronic pain, improve the safety and effectiveness of pain treatment, and reduce the risks associated with long-term opioid therapy, including opioid use disorder, overdose, and death. CDC has provided a checklist for prescribing opioids for chronic pain ( http://stacks.cdc.gov/view/cdc/38025) as well as a website ( http://www.cdc.gov/drugoverdose/prescribingresources.html) with additional tools to guide clinicians in implementing the recommendations.
How often should you evaluate opioids?
Clinicians should evaluate benefits and harms with patients within 1 to 4 weeks of starting opioid therapy for chronic pain or of dose escalation. Clinicians should evaluate benefits and harms of continued therapy with patients every 3 months or more frequently.
What is the opioid prescribed for?
Background. Opioids are commonly prescribed for pain. An estimated 20% of patients presenting to physician offices with noncancer pain symptoms or pain-related diagnoses (including acute and chronic pain) receive an opioid prescription ( 1 ).
How many people were prescribed opioids in 2005?
On the basis of data available from health systems, researchers estimate that 9.6–11.5 million adults, or approximately 3%–4% of the adult U.S. population, were prescribed long-term opioid therapy in 2005 ( 15 ). Opioid pain medication use presents serious risks, including overdose and opioid use disorder.
Is opioid pain medication overprescribed?
Across specialties, physicians believe that opioid pain medication can be effective in controlling pain, that addiction is a common consequence of prolonged use, and that long-term opioid therapy often is overprescribed for patients with chronic noncancer pain ( 27 ).
Can opioids cause a nonfatal overdose?
Although studies were not identified that directly addressed the risk for overdose among patients with prior nonfatal overdose who are prescribed opioids, based on clinical experience, experts thought that prior nonfatal overdose would substantially increase risk for future nonfatal or fatal opioid overdose. If patients experience nonfatal opioid overdose, clinicians should work with them to reduce opioid dosage and to discontinue opioids when possible (see Recommendation 7). If clinicians continue opioid therapy for chronic pain outside of active cancer, palliative, and end-of-life care in patients with prior opioid overdose, they should discuss increased risks for overdose with patients, carefully consider whether benefits of opioids outweigh substantial risks, and incorporate strategies to mitigate risk into the management plan, such as considering offering naloxone (see Offering Naloxone to Patients When Factors That Increase Risk for Opioid-Related Harms Are Present) and increasing frequency of monitoring (see Recommendation 7) when opioids are prescribed.
Does naloxone help with respiratory depression?
Naloxone is an opioid antagonist that can reverse severe respiratory depression; its administration by lay persons, such as friends and family of persons who experience opioid overdose, can save lives. Naloxone precipitates acute withdrawal among patients physically dependent on opioids. Serious adverse effects, such as pulmonary edema, cardiovascular instability, and seizures, have been reported but are rare at doses consistent with labeled use for opioid overdose ( 210 ). The contextual evidence review did not find any studies on effectiveness of prescribing naloxone for overdose prevention among patients prescribed opioids for chronic pain. However, there is evidence for effectiveness of naloxone provision in preventing opioid-related overdose death at the community level through community-based distribution (e.g., through overdose education and naloxone distribution programs in community service agencies) to persons at risk for overdose (mostly due to illicit opiate use), and it is plausible that effectiveness would be observed when naloxone is provided in the clinical setting as well. Experts agreed that it is preferable not to initiate opioid treatment when factors that increase risk for opioid-related harms are present. Opinions diverged about the likelihood of naloxone being useful to patients and the circumstances under which it should be offered. However, most experts agreed that clinicians should consider offering naloxone when prescribing opioids to patients at increased risk for overdose, including patients with a history of overdose, patients with a history of substance use disorder, patients taking benzodiazepines with opioids (see Recommendation 11), patients at risk for returning to a high dose to which they are no longer tolerant (e.g., patients recently released from prison), and patients taking higher dosages of opioids (≥50 MME/day). Practices should provide education on overdose prevention and naloxone use to patients receiving naloxone prescriptions and to members of their households. Experts noted that naloxone co-prescribing can be facilitated by clinics or practices with resources to provide naloxone training and by collaborative practice models with pharmacists. Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org#N#external icon#N#.
